Back
Poster Session A
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (0034–0044) Pediatric Rheumatology – Basic Science Poster
0041: Modeling Juvenile Dermatomyositis with Engineered Human Skeletal Muscle: Effects of Type I Interferonβ and Janus Kinase Inhibitors
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Lauren Covert, MD
Duke University
Durham, NC, United States
Abstract Poster Presenter(s)
Lauren Covert1, George Truskey2 and Jeffrey Dvergsten1, 1Duke University Hospital, Durham, NC, 2Duke University, Durham, NC
Background/Purpose: Upregulation of Type I interferons (IFN I), including IFNβ, is a hallmark of adult and juvenile dermatomyositis (JDM), but its role in pathogenesis is not clearly understood. Since IFN I activates the Janus kinase (JAK)-signal transducer and transcription (STAT) pathway, JAK inhibitors, including baricitinib (JAK 1/2 inhibitor) and tofacitinib (JAK 1/2/3 inhibitor), have therapeutic potential for JDM. Lack of adequate clinical trials, difficulty in obtaining routine JDM muscle biopsies, and absence of a disease model hinder understanding pathologic triggers and delay development of needed therapies. Using an in vitro 3D biomimetic construct derived from healthy pediatric muscle (termed "myobundles"), preliminary work demonstrated IFNβ-associated decrease in tetanus contractile force, slowed twitch kinetics, and paradoxically decreased fatigue.
Methods: As seen in Figure 1, myogenic cells isolated from 2 healthy pediatric donors were cultured and used to create donor-specific myobundles based on established protocols (Madden, 2015). After growth and differentiation, myobundles were exposed to 0 (control condition) or 20 ng/mL IFNβ for a total of 10 days. Tofacitinib 1 uM (suspended in H2O) and baricitinib 500 nM (suspended in DMSO 0.01%) were introduced to culture media during the last 6 days of IFNβ exposure. Control myobundles were treated with dimethyl sulfoxide (DMSO) 0.01% at this same timepoint. On day 21 after myobundle creation, contractile force after twitch (1 Hz for 10 ms), tetanus (20 Hz for 1 s), and fatigue (20 Hz for 30 s) electrical stimulation was measured. To assess kinetics, time to reach maximum force and half-relax were measured after twitch stimulation. Fatigue percentage was determined by reduction in force after fatigue stimulation. Data was analyzed using one-way ANOVA with multiple post hoc comparisons.
Results: Tofacitinib and baricitinib reversed IFNβ-associated twitch force reduction in one donor and significantly increased twitch force in the other donor (Fig. 2, top). IFNβ-associated reduction in tetanus force was reversed by tofacitinib in both donors and by baricitinib in one donor (Fig. 2, bottom). Time to maximum twitch force was increased in IFNβ-exposed myobundles in one donor (p< 0.0001). This increase normalized after tofacitnib and baricitinib. Twitch time to half-relax increased after IFNβ. This normalized after JAK inhibition (Fig. 3, top). IFNβ-associated decrease in fatigue did not reverse after tofacitinib or baricitinib treatment (Fig. 3, bottom).
Conclusion: JAK inhibitors tofacitinib and baricitinib reversed IFNβ-associated decrease in tetanus contractile force and slowed twitch kinetics but did not change muscle fatigue for 2 donors, suggesting donor-specific response. This 3D engineered tissue platform is a novel model to further develop for researching JDM pathogenesis and assessing therapeutic efficacy.
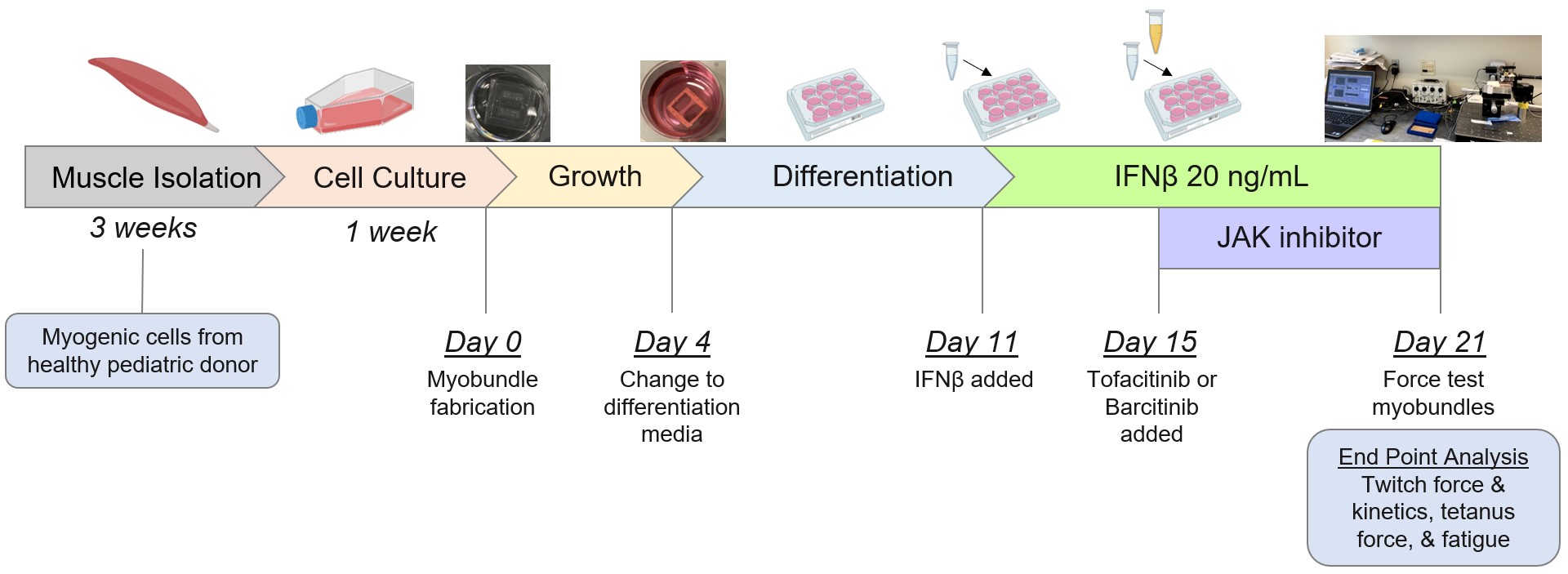 Figure 1. Experimental protocol and timeline.
Figure 1. Experimental protocol and timeline.
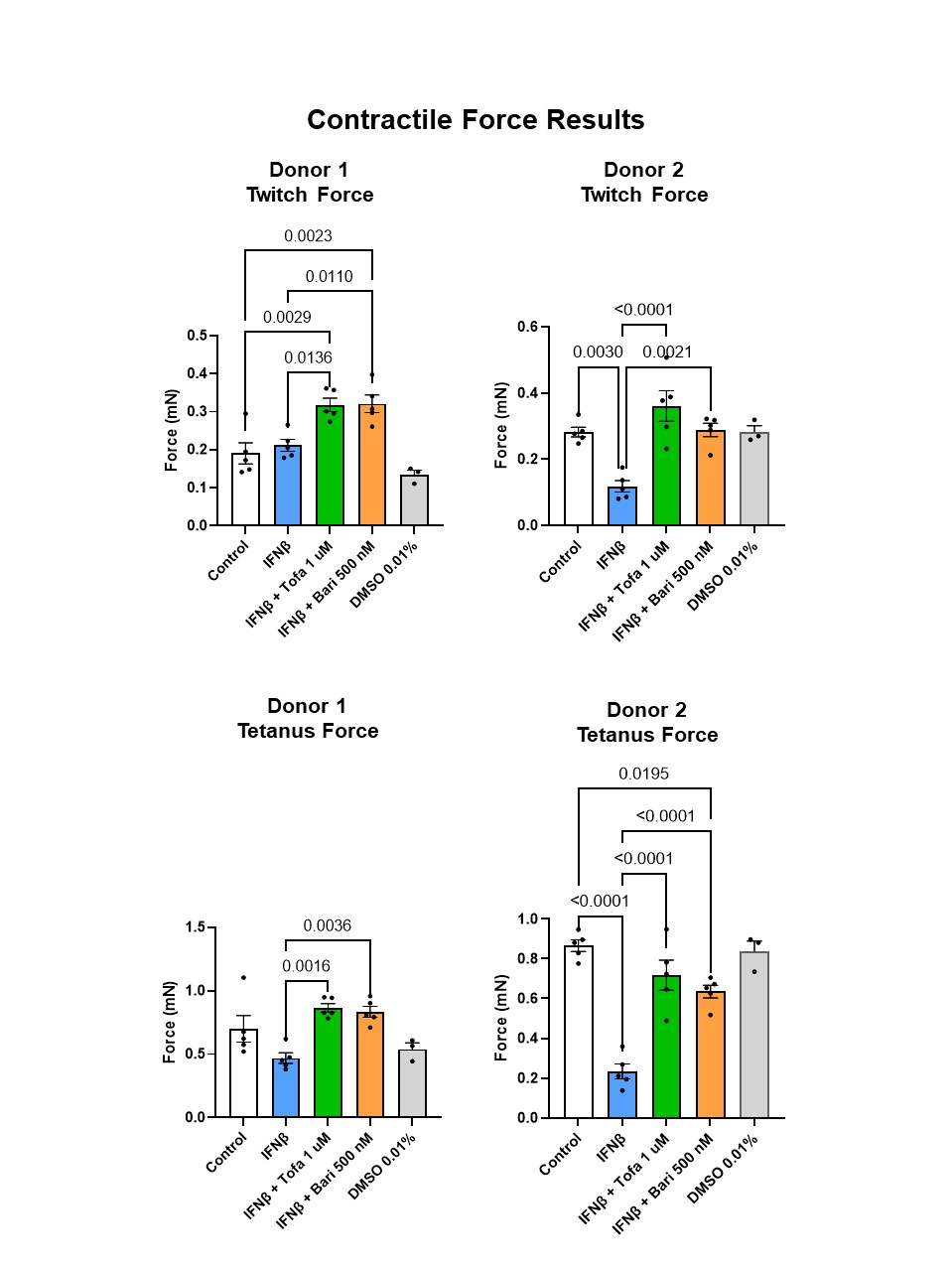 Figure 2. Twitch (top) and tetanus (bottom) force results across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
Figure 2. Twitch (top) and tetanus (bottom) force results across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
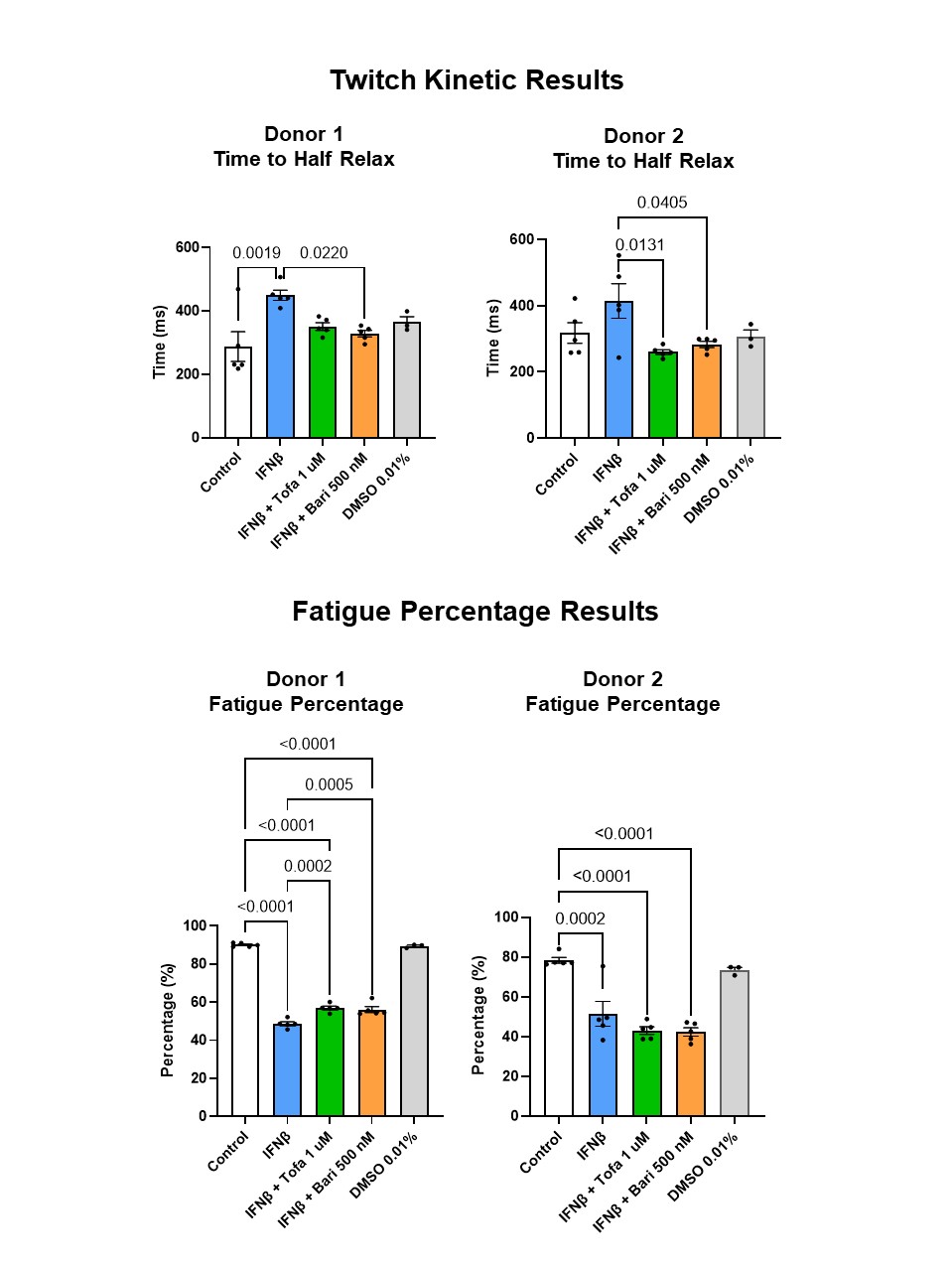 Figure 3. Twitch time to half relax (top) and fatigue percentage (bottom) across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
Figure 3. Twitch time to half relax (top) and fatigue percentage (bottom) across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
Disclosures: L. Covert, None; G. Truskey, None; J. Dvergsten, None.
Background/Purpose: Upregulation of Type I interferons (IFN I), including IFNβ, is a hallmark of adult and juvenile dermatomyositis (JDM), but its role in pathogenesis is not clearly understood. Since IFN I activates the Janus kinase (JAK)-signal transducer and transcription (STAT) pathway, JAK inhibitors, including baricitinib (JAK 1/2 inhibitor) and tofacitinib (JAK 1/2/3 inhibitor), have therapeutic potential for JDM. Lack of adequate clinical trials, difficulty in obtaining routine JDM muscle biopsies, and absence of a disease model hinder understanding pathologic triggers and delay development of needed therapies. Using an in vitro 3D biomimetic construct derived from healthy pediatric muscle (termed "myobundles"), preliminary work demonstrated IFNβ-associated decrease in tetanus contractile force, slowed twitch kinetics, and paradoxically decreased fatigue.
- Aim: Determine functional effects of tofacitinib and baricitinib on myobundles exposed concurrently to IFNβ.
- Hypothesis: JAK inhibitors normalize IFNβ-associated decrease in tetanus contractile force but do not reverse changes in twitch kinetics or fatigue.
Methods: As seen in Figure 1, myogenic cells isolated from 2 healthy pediatric donors were cultured and used to create donor-specific myobundles based on established protocols (Madden, 2015). After growth and differentiation, myobundles were exposed to 0 (control condition) or 20 ng/mL IFNβ for a total of 10 days. Tofacitinib 1 uM (suspended in H2O) and baricitinib 500 nM (suspended in DMSO 0.01%) were introduced to culture media during the last 6 days of IFNβ exposure. Control myobundles were treated with dimethyl sulfoxide (DMSO) 0.01% at this same timepoint. On day 21 after myobundle creation, contractile force after twitch (1 Hz for 10 ms), tetanus (20 Hz for 1 s), and fatigue (20 Hz for 30 s) electrical stimulation was measured. To assess kinetics, time to reach maximum force and half-relax were measured after twitch stimulation. Fatigue percentage was determined by reduction in force after fatigue stimulation. Data was analyzed using one-way ANOVA with multiple post hoc comparisons.
Results: Tofacitinib and baricitinib reversed IFNβ-associated twitch force reduction in one donor and significantly increased twitch force in the other donor (Fig. 2, top). IFNβ-associated reduction in tetanus force was reversed by tofacitinib in both donors and by baricitinib in one donor (Fig. 2, bottom). Time to maximum twitch force was increased in IFNβ-exposed myobundles in one donor (p< 0.0001). This increase normalized after tofacitnib and baricitinib. Twitch time to half-relax increased after IFNβ. This normalized after JAK inhibition (Fig. 3, top). IFNβ-associated decrease in fatigue did not reverse after tofacitinib or baricitinib treatment (Fig. 3, bottom).
Conclusion: JAK inhibitors tofacitinib and baricitinib reversed IFNβ-associated decrease in tetanus contractile force and slowed twitch kinetics but did not change muscle fatigue for 2 donors, suggesting donor-specific response. This 3D engineered tissue platform is a novel model to further develop for researching JDM pathogenesis and assessing therapeutic efficacy.
 Figure 1. Experimental protocol and timeline.
Figure 1. Experimental protocol and timeline. Figure 2. Twitch (top) and tetanus (bottom) force results across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
Figure 2. Twitch (top) and tetanus (bottom) force results across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide. Figure 3. Twitch time to half relax (top) and fatigue percentage (bottom) across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.
Figure 3. Twitch time to half relax (top) and fatigue percentage (bottom) across 2 donors. Mean +/- SEM depicted. Tofa = tofacitinib; Bari = baricitinib; DMSO = dimethyl sulfoxide.Disclosures: L. Covert, None; G. Truskey, None; J. Dvergsten, None.

