Back
Poster Session A
Epidemiology, health policy and outcomes
Session: (0187–0213) Patient Outcomes, Preferences, and Attitudes Poster I
0208: Cutaneous Dermatomyositis Disease Area and Severity Index Activity Score (CDASI-A) and Associated Patient-Reported Outcomes and Biomarkers in a Phase 2 Clinical Trial in Dermatomyositis (DM)
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JD
Joshua Dan, BA
Icahn School of Medicine at Mount Sinai
New York, NY, United States
Abstract Poster Presenter(s)
Josh Dan1, Jay Patel2, Grant Sprow3, DeAnna Diaz4, Nilesh Kodali5, Rui Feng6, Barbara White7 and Victoria Werth8, 1Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, 2Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PN, 3Albert Einstein College of Medicine, Philadelphia, PA, 4Philadelphia College of Medicine, Philadelphia, PA, 5New Jersey Medical School, Coppell, TX, 6University of Pennsylvania, Philadelphia, 7SFJ Pharmaceuticals, Towson, MD, 8University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA
Background/Purpose: Retrospective reviews of clinical databases from two sites have identified strong relationships between patient-reported outcomes and skin activity in DM, as measured by CDASI-A. No studies validate these associations in a controlled setting. Additionally, the relationship between the Patient Reported Outcomes Measurement Information System-29 (PROMIS) Short Form and skin activity in DM has not been assessed. Previous investigations have demonstrated a correlation between IL-31 and itch in DM. IFN-β and IFN-γ are known type I and II interferons, which are critical drivers of DM pathogenesis. We assessed correlations between changes in CDASI-A, quality of life (QoL) outcomes, and biomarkers of disease activity in a double-blind, randomized, placebo-controlled clinical tria.
Methods: Data were retrospectively collected from five visits of a Phase 2 trial evaluating lenabasum, a cannabinoid receptor type 2 agonist, for treatment of DM. Quality of life assessments collected in the trial included Patient Global Assessment (PtGA) scores, PROMIS domains, and Skindex domains. Skindex question 10, regarding itch, was included as a separate domain. Physician Global Assessment scores were evaluated. Cytokines measured in skin samples using immunohistochemistry/polymerase chain reaction collected at baseline and week 12 were assessed for predictors of CDASI-A response and association with disease activity. Analyses used linear mixed effect models to account for within-subject variability and repeated measures, where applicable. Analyses were performed without regard to treatment arm, to correlate CDASI, QoL, and biomarkers among all subjects.
Results: Clinical trial data from 22 subjects at 110 visits and cytokine data from pre- and end-of-treatment skin biopsies from 12 of these subjects were analyzed. Correlations between CDASI-A and Skindex-29, PROMIS, PtGA and PGA are listed in Table 1. Improvement in CDASI-A correlated with reduction in IL-31 protein area (p = 0.047). CDASI-A improvement also correlated with reduction in IFN-β (p = 0.081) and IFN-γ (p = 0.134) protein areas in skin biopsies, but p > 0.05.
Conclusion: The results extend previous similar findings in observational databases and support the use of CDASI-A as an efficacy outcome in DM clinical trials that reflects both physician- and patient-assessed changes in skin disease. These results show for the first time in a clinical study that changes in CDASI-A correlate strongly with Skindex domains and PtGA scores, all well-established measures of QoL in DM patients. Improvement in CDASI-A correlated with all PROMIS domains except physical function, though Skindex-29 demonstrated comparatively stronger p-values and better responsiveness to CDASI-A. This finding may suggest Skindex-29 may more accurately capture patient reports of skin disease, compared to PROMIS domains. The directionally correct correlations of change in CDASI-A with changes in IL-31, a cytokine previously associated with itch in DM, IFN-β, and IFN-γ support the role of these cytokines in the pathogenesis of skin disease in DM.
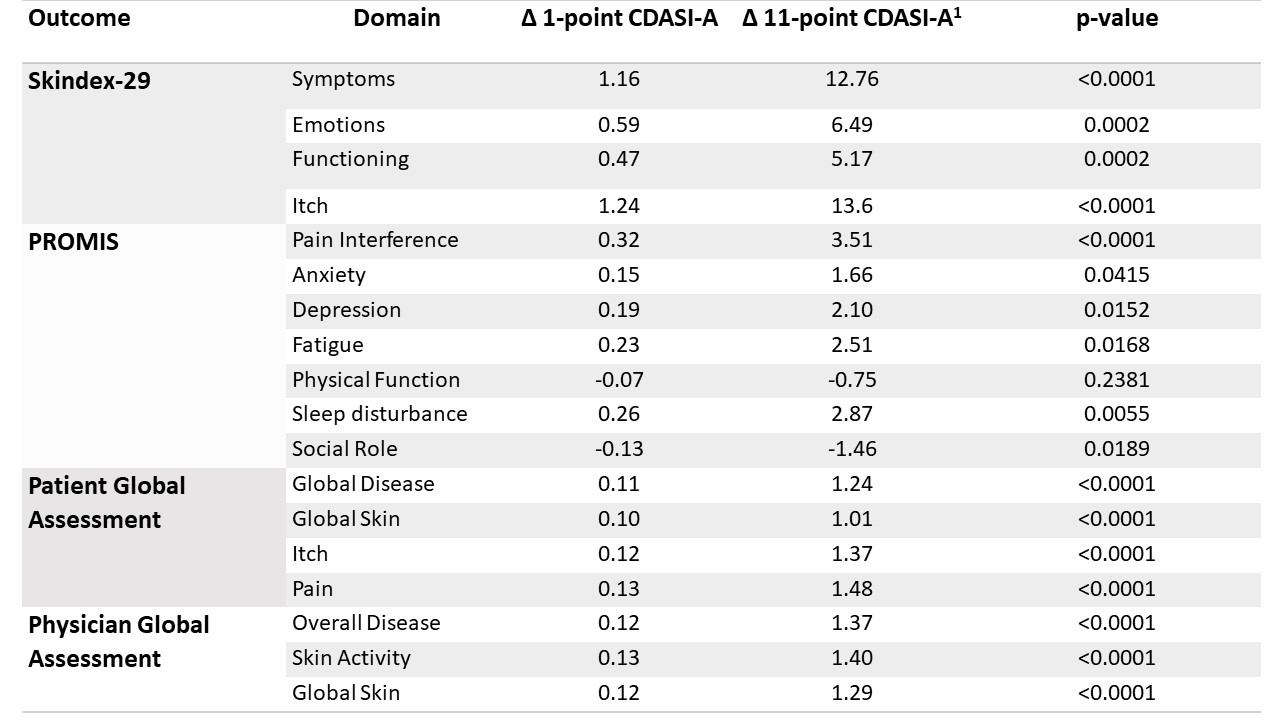 Table 1. Linear mixed effects models assessing the relationship between each domain of Skindex-29, PROMIS, Patient Global Assessment, Physician Global Assessment, and CDASI-A.
Table 1. Linear mixed effects models assessing the relationship between each domain of Skindex-29, PROMIS, Patient Global Assessment, Physician Global Assessment, and CDASI-A.
1Associated change in patient reported outcome for an 11-point change, or important change, in CDASI-A.
Disclosures: J. Dan, None; J. Patel, None; G. Sprow, None; D. Diaz, None; N. Kodali, None; R. Feng, None; B. White, Corbus Pharmaceuticals; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela.
Background/Purpose: Retrospective reviews of clinical databases from two sites have identified strong relationships between patient-reported outcomes and skin activity in DM, as measured by CDASI-A. No studies validate these associations in a controlled setting. Additionally, the relationship between the Patient Reported Outcomes Measurement Information System-29 (PROMIS) Short Form and skin activity in DM has not been assessed. Previous investigations have demonstrated a correlation between IL-31 and itch in DM. IFN-β and IFN-γ are known type I and II interferons, which are critical drivers of DM pathogenesis. We assessed correlations between changes in CDASI-A, quality of life (QoL) outcomes, and biomarkers of disease activity in a double-blind, randomized, placebo-controlled clinical tria.
Methods: Data were retrospectively collected from five visits of a Phase 2 trial evaluating lenabasum, a cannabinoid receptor type 2 agonist, for treatment of DM. Quality of life assessments collected in the trial included Patient Global Assessment (PtGA) scores, PROMIS domains, and Skindex domains. Skindex question 10, regarding itch, was included as a separate domain. Physician Global Assessment scores were evaluated. Cytokines measured in skin samples using immunohistochemistry/polymerase chain reaction collected at baseline and week 12 were assessed for predictors of CDASI-A response and association with disease activity. Analyses used linear mixed effect models to account for within-subject variability and repeated measures, where applicable. Analyses were performed without regard to treatment arm, to correlate CDASI, QoL, and biomarkers among all subjects.
Results: Clinical trial data from 22 subjects at 110 visits and cytokine data from pre- and end-of-treatment skin biopsies from 12 of these subjects were analyzed. Correlations between CDASI-A and Skindex-29, PROMIS, PtGA and PGA are listed in Table 1. Improvement in CDASI-A correlated with reduction in IL-31 protein area (p = 0.047). CDASI-A improvement also correlated with reduction in IFN-β (p = 0.081) and IFN-γ (p = 0.134) protein areas in skin biopsies, but p > 0.05.
Conclusion: The results extend previous similar findings in observational databases and support the use of CDASI-A as an efficacy outcome in DM clinical trials that reflects both physician- and patient-assessed changes in skin disease. These results show for the first time in a clinical study that changes in CDASI-A correlate strongly with Skindex domains and PtGA scores, all well-established measures of QoL in DM patients. Improvement in CDASI-A correlated with all PROMIS domains except physical function, though Skindex-29 demonstrated comparatively stronger p-values and better responsiveness to CDASI-A. This finding may suggest Skindex-29 may more accurately capture patient reports of skin disease, compared to PROMIS domains. The directionally correct correlations of change in CDASI-A with changes in IL-31, a cytokine previously associated with itch in DM, IFN-β, and IFN-γ support the role of these cytokines in the pathogenesis of skin disease in DM.
 Table 1. Linear mixed effects models assessing the relationship between each domain of Skindex-29, PROMIS, Patient Global Assessment, Physician Global Assessment, and CDASI-A.
Table 1. Linear mixed effects models assessing the relationship between each domain of Skindex-29, PROMIS, Patient Global Assessment, Physician Global Assessment, and CDASI-A. 1Associated change in patient reported outcome for an 11-point change, or important change, in CDASI-A.
Disclosures: J. Dan, None; J. Patel, None; G. Sprow, None; D. Diaz, None; N. Kodali, None; R. Feng, None; B. White, Corbus Pharmaceuticals; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela.

