Back
Poster Session A
Diversity, inclusion and racial disparities
Session: (0085–0122) Healthcare Disparities in Rheumatology Poster
0095: Association of Urban vs. Rural Residence with New DMARD Initiation in US Veterans with Active Rheumatoid Arthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LD
Luke Desilet, DO
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Luke Desilet1, Harlan Sayles1, Punyasha Roul2, Jennifer Barton3, Gail Kerr4, Kaleb Michaud1, Ted Mikuls5 and Bryant England1, 1University of Nebraska Medical Center, Omaha, NE, 2UNMC, Omaha, NE, 3VA Portland Health Care System/OHSU, Portland, OR, 4Washington DC VAMC/Georgetown and Howard Universities, Washington, DC, 5Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: While a treat-to-target strategy is endorsed by the ACR RA Treatment Guidelines, many patient, social, and healthcare system factors make implementation of this approach challenging in real-world clinical settings. Rural residence has been associated with disparities in healthcare-related outcomes in both rheumatic diseases and other chronic conditions. Thus, we examined whether urban vs. rural residence was associated with the frequency of new DMARD initiations among US Veterans with persistently active RA.
Methods: We performed a cohort study of US Veterans with RA in the Veterans Affairs Rheumatoid Arthritis (VARA) registry between 2003 and 2020. We identified patients with persistently active RA (2 consecutive visits with moderate or high disease activity by DAS28) who did not initiate a new DMARD between visits. Urban or rural residence was based on Veterans Health Administration (VHA) maintained residential address urban/rural categorization. The primary outcome was initiation of a new DMARD (not utilized at the last 2 visits) within 1 year of the index visit, defined as the second date of moderate-to-high disease activity. Secondary outcomes were new initiations of specific DMARD categories (conventional synthetic DMARDs, TNFi biologic DMARDs, and non-TNFi biologic/targeted synthetic DMARDs). Multivariable logistic regression models included age, sex, race, education, smoking status, anti-CCP status, RA disease duration, BMI, Rheumatic Disease Comorbidity Index (RDCI), VARA site, calendar year (2003-2010 vs. 2011-2020), baseline DAS28, number of prior DMARDs, and prednisone use.
Results: We identified 1,461 patients with persistently active RA who did not recently initiate a new DMARD during our observation period. Patients were predominantly male (89%) and had a mean (SD) age of 71.4 (10.2) years (Table 1). Overall, 902 (62%) patients resided in urban areas and 559 (38%) resided in rural areas. Rural patients were more likely than urban counterparts to be male, white, current smokers and they had more baseline prednisone and prior DMARD use (Table 1). The proportion of patients initiating any new DMARD over the following year was similar between urban (n=363, 40%) and rural (n=234, 42%) patients. Following multivariable adjustment, no association was observed between urban vs. rural residence and new DMARD initiation (aOR 1.19 [0.87, 1.64]; Table 2). Similarly, no significant associations were observed between urban vs. rural residence and initiation of specific DMARD categories (conventional synthetic DMARDs, TNFi biologic DMARDs, and non-TNFi biologic/targeted synthetic DMARDs) after adjustment for covariates (Table 3).
Conclusion: Using real-world data from a multicenter observational RA cohort, we found that less than half of US Veterans with RA were prescribed a new DMARD in the setting of persistently active disease. Rates of new DMARD initiation did not differ between those in urban and rural areas, suggesting that within the VHA system, urban-rural health disparities are not responsible for difficulties implementing a treat-to-target strategy. Additional interventions are needed to improve implementation of treat-to-target strategies for RA regardless of rurality.
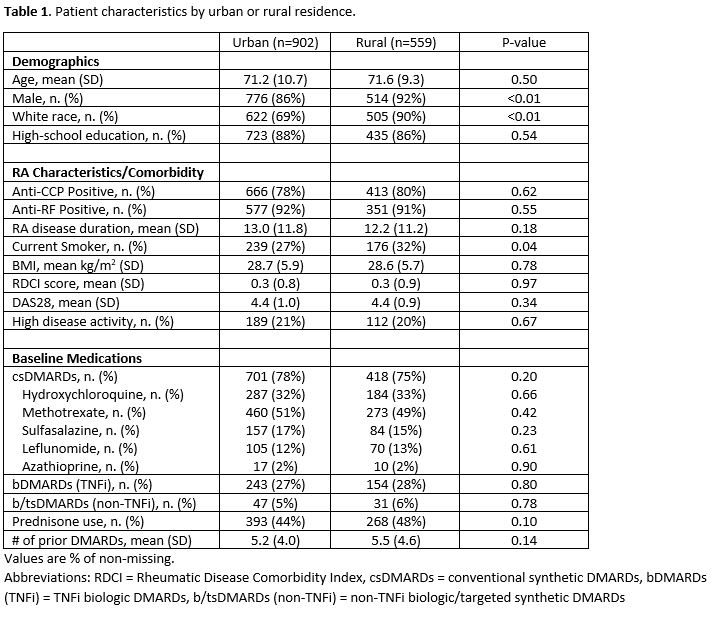 Patient characteristics by urban or rural residence.
Patient characteristics by urban or rural residence.
 Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA
Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA
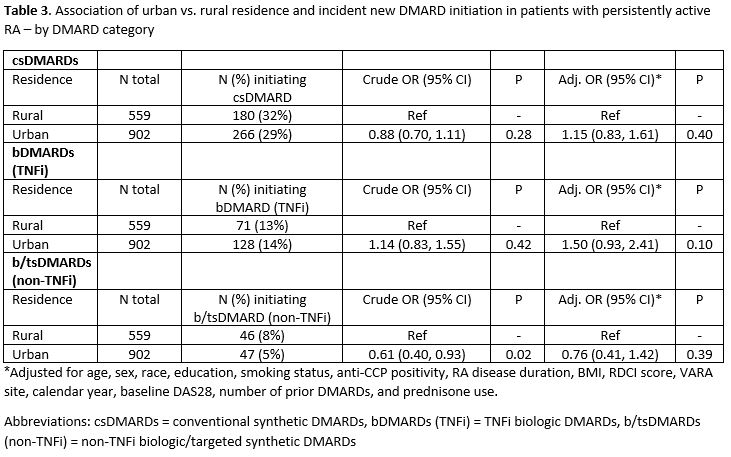 Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA – by DMARD category
Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA – by DMARD category
Disclosures: L. Desilet, None; H. Sayles, None; P. Roul, None; J. Barton, None; G. Kerr, Pfizer, Janssen; K. Michaud, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; B. England, Boehringer-Ingelheim.
Background/Purpose: While a treat-to-target strategy is endorsed by the ACR RA Treatment Guidelines, many patient, social, and healthcare system factors make implementation of this approach challenging in real-world clinical settings. Rural residence has been associated with disparities in healthcare-related outcomes in both rheumatic diseases and other chronic conditions. Thus, we examined whether urban vs. rural residence was associated with the frequency of new DMARD initiations among US Veterans with persistently active RA.
Methods: We performed a cohort study of US Veterans with RA in the Veterans Affairs Rheumatoid Arthritis (VARA) registry between 2003 and 2020. We identified patients with persistently active RA (2 consecutive visits with moderate or high disease activity by DAS28) who did not initiate a new DMARD between visits. Urban or rural residence was based on Veterans Health Administration (VHA) maintained residential address urban/rural categorization. The primary outcome was initiation of a new DMARD (not utilized at the last 2 visits) within 1 year of the index visit, defined as the second date of moderate-to-high disease activity. Secondary outcomes were new initiations of specific DMARD categories (conventional synthetic DMARDs, TNFi biologic DMARDs, and non-TNFi biologic/targeted synthetic DMARDs). Multivariable logistic regression models included age, sex, race, education, smoking status, anti-CCP status, RA disease duration, BMI, Rheumatic Disease Comorbidity Index (RDCI), VARA site, calendar year (2003-2010 vs. 2011-2020), baseline DAS28, number of prior DMARDs, and prednisone use.
Results: We identified 1,461 patients with persistently active RA who did not recently initiate a new DMARD during our observation period. Patients were predominantly male (89%) and had a mean (SD) age of 71.4 (10.2) years (Table 1). Overall, 902 (62%) patients resided in urban areas and 559 (38%) resided in rural areas. Rural patients were more likely than urban counterparts to be male, white, current smokers and they had more baseline prednisone and prior DMARD use (Table 1). The proportion of patients initiating any new DMARD over the following year was similar between urban (n=363, 40%) and rural (n=234, 42%) patients. Following multivariable adjustment, no association was observed between urban vs. rural residence and new DMARD initiation (aOR 1.19 [0.87, 1.64]; Table 2). Similarly, no significant associations were observed between urban vs. rural residence and initiation of specific DMARD categories (conventional synthetic DMARDs, TNFi biologic DMARDs, and non-TNFi biologic/targeted synthetic DMARDs) after adjustment for covariates (Table 3).
Conclusion: Using real-world data from a multicenter observational RA cohort, we found that less than half of US Veterans with RA were prescribed a new DMARD in the setting of persistently active disease. Rates of new DMARD initiation did not differ between those in urban and rural areas, suggesting that within the VHA system, urban-rural health disparities are not responsible for difficulties implementing a treat-to-target strategy. Additional interventions are needed to improve implementation of treat-to-target strategies for RA regardless of rurality.
 Patient characteristics by urban or rural residence.
Patient characteristics by urban or rural residence. Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA
Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA – by DMARD category
Association of urban vs. rural residence and incident new DMARD initiation in patients with persistently active RA – by DMARD categoryDisclosures: L. Desilet, None; H. Sayles, None; P. Roul, None; J. Barton, None; G. Kerr, Pfizer, Janssen; K. Michaud, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; B. England, Boehringer-Ingelheim.

