Back
Poster Session A
Epidemiology, health policy and outcomes
Session: (0187–0213) Patient Outcomes, Preferences, and Attitudes Poster I
0198: Patient Perspectives on Janus Kinase Inhibitor Use in the Treatment of Inflammatory Arthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- Sd
Savia de Souza, BSc
Independent
Greater London, United Kingdom
Abstract Poster Presenter(s)
Savia de Souza1, Andrew Bassett2, Ruth Williams2 and Elena Nikiphorou3, 1Independent, London, United Kingdom, 2King's College London, London, United Kingdom, 3Leiden University Medical Center & King's College London, London, United Kingdom
Background/Purpose: Janus kinase inhibitors (JAKi) have relatively recently been licensed to treat inflammatory arthritis (IA); specifically rheumatoid arthritis (RA) and psoriatic arthritis (PsA). They differ from biologics, often currently prescribed, by targeting a different part of the inflammatory pathway and being taken orally. The general lack of data on patient perspective on JAKi use formed the rationale for this study.
Methods: A 24-item anonymous survey, with a mix of closed and open-ended questions, was co-designed with 2 Patient Research Partners with IA. The survey was distributed online in October 2021 via patient charities and social media. Inclusion criteria were UK-based adult patients with a diagnosis of RA/PsA who are currently on a biologic/JAKi ( > 6 months) or have previously taken a JAKi. Data from completed surveys by patients who met the inclusion criteria were imported into SPSS Statistics 27 and NVivo 12 Pro to aid descriptive statistical and thematic analyses.
Results: Of the 175 surveys received, 81% were eligible for the final analysis (due to some not meeting the inclusion criteria, incomplete and duplicate surveys). See Table 1 for patient demographics.
Cross-tabulation showed 39% of JAKi patients being 'very satisfied' overall with their medication, compared with 25% on biologics. See Table 2 for free text response analysis with regard to overall satisfaction with arthritis medication. Seventeen patients (12%) reported stopping a JAKi; almost half of them (47%) stopped within 1-3 months. Free text response analysis showed inefficacy (10 references) and adverse effects (9 references) to be the most common reasons for stopping JAKi.
Overall awareness of JAKi reported by patients was 'somewhat aware' (40%), followed by 'not aware/not very aware/not aware at all' (33%) and 'very aware' (26%). The 3 most reported sources of information on JAKi (multiple options allowed) were a rheumatologist (50%), patient organisation (31%) and the internet (28%).
Patients currently on a biologic were asked if they would prefer an oral therapy instead (such as JAKi). The majority reported 'don't know' (45%), followed by 'yes' (27%) and 'no' (14%). Fifty-nine additional comments were left at the end of the survey - see Table 3 for themes.
Conclusion: IA patients on JAKi were more likely to be very satisfied overall on their arthritis medication than those on biologics. The most common reasons for stopping a JAKi were inefficacy and adverse effects. Most patients have some knowledge of JAKi, mainly from their rheumatologist. Nearly half of patients on biologics were undecided as to whether they would prefer an oral therapy, with concerns over recent warnings about JAKi. However, patients currently on JAKi appreciated its short half-life and convenience.
.jpg) Table 1 - Patient demographics
Table 1 - Patient demographics
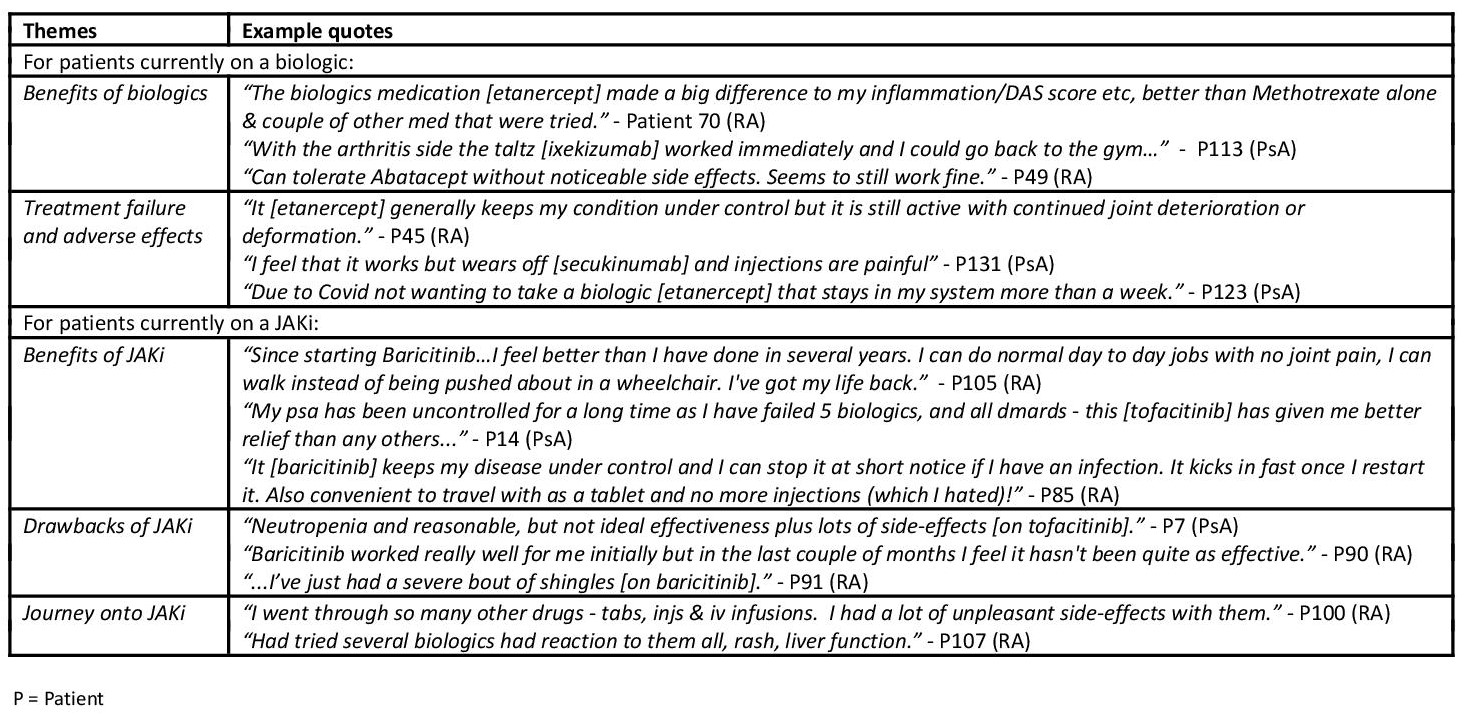 Table 2 - Overall satisfaction with current arthritis medication
Table 2 - Overall satisfaction with current arthritis medication
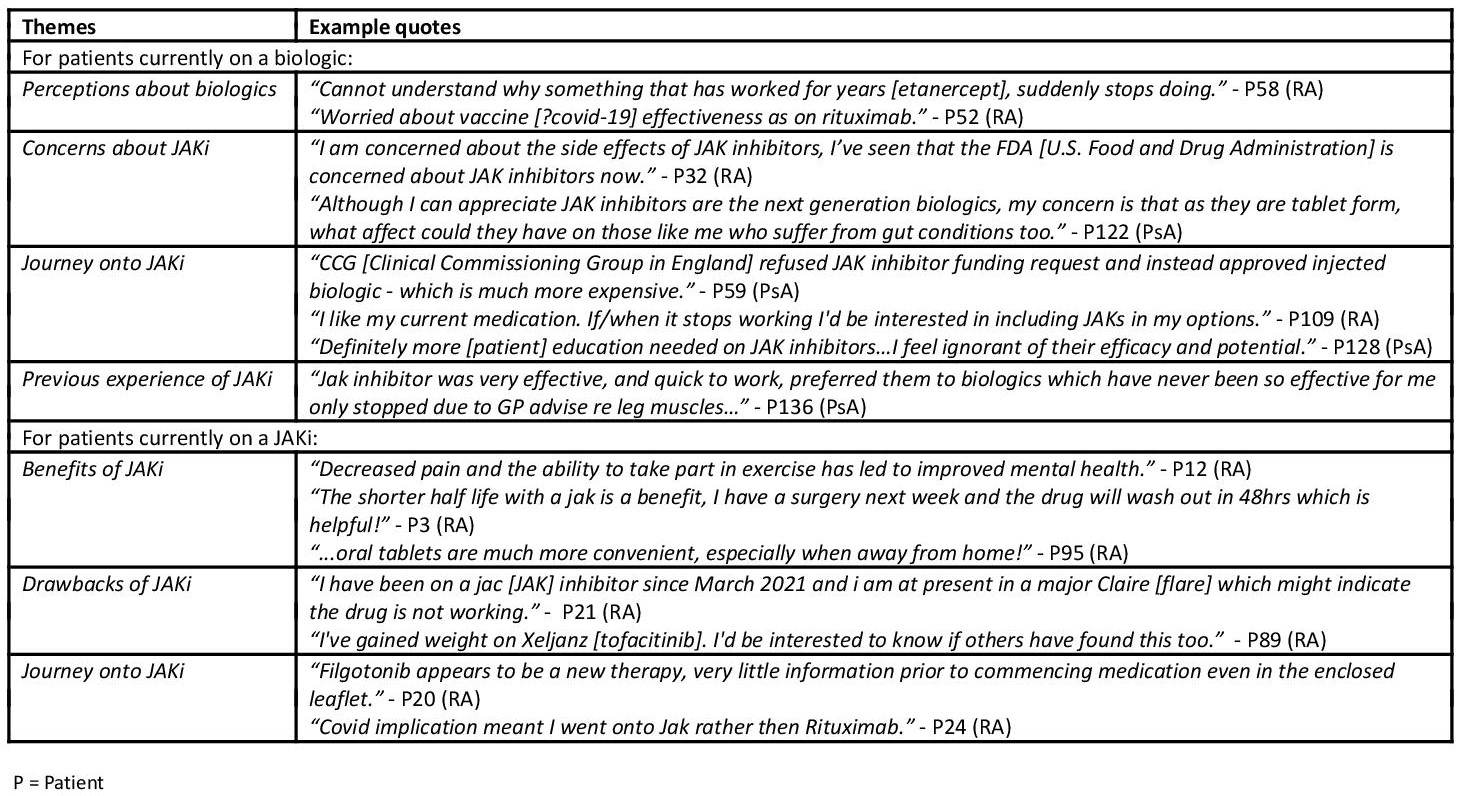 Table 3 - Additional comments
Table 3 - Additional comments
Disclosures: S. de Souza, None; A. Bassett, None; R. Williams, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.
Background/Purpose: Janus kinase inhibitors (JAKi) have relatively recently been licensed to treat inflammatory arthritis (IA); specifically rheumatoid arthritis (RA) and psoriatic arthritis (PsA). They differ from biologics, often currently prescribed, by targeting a different part of the inflammatory pathway and being taken orally. The general lack of data on patient perspective on JAKi use formed the rationale for this study.
Methods: A 24-item anonymous survey, with a mix of closed and open-ended questions, was co-designed with 2 Patient Research Partners with IA. The survey was distributed online in October 2021 via patient charities and social media. Inclusion criteria were UK-based adult patients with a diagnosis of RA/PsA who are currently on a biologic/JAKi ( > 6 months) or have previously taken a JAKi. Data from completed surveys by patients who met the inclusion criteria were imported into SPSS Statistics 27 and NVivo 12 Pro to aid descriptive statistical and thematic analyses.
Results: Of the 175 surveys received, 81% were eligible for the final analysis (due to some not meeting the inclusion criteria, incomplete and duplicate surveys). See Table 1 for patient demographics.
Cross-tabulation showed 39% of JAKi patients being 'very satisfied' overall with their medication, compared with 25% on biologics. See Table 2 for free text response analysis with regard to overall satisfaction with arthritis medication. Seventeen patients (12%) reported stopping a JAKi; almost half of them (47%) stopped within 1-3 months. Free text response analysis showed inefficacy (10 references) and adverse effects (9 references) to be the most common reasons for stopping JAKi.
Overall awareness of JAKi reported by patients was 'somewhat aware' (40%), followed by 'not aware/not very aware/not aware at all' (33%) and 'very aware' (26%). The 3 most reported sources of information on JAKi (multiple options allowed) were a rheumatologist (50%), patient organisation (31%) and the internet (28%).
Patients currently on a biologic were asked if they would prefer an oral therapy instead (such as JAKi). The majority reported 'don't know' (45%), followed by 'yes' (27%) and 'no' (14%). Fifty-nine additional comments were left at the end of the survey - see Table 3 for themes.
Conclusion: IA patients on JAKi were more likely to be very satisfied overall on their arthritis medication than those on biologics. The most common reasons for stopping a JAKi were inefficacy and adverse effects. Most patients have some knowledge of JAKi, mainly from their rheumatologist. Nearly half of patients on biologics were undecided as to whether they would prefer an oral therapy, with concerns over recent warnings about JAKi. However, patients currently on JAKi appreciated its short half-life and convenience.
.jpg) Table 1 - Patient demographics
Table 1 - Patient demographics Table 2 - Overall satisfaction with current arthritis medication
Table 2 - Overall satisfaction with current arthritis medication Table 3 - Additional comments
Table 3 - Additional commentsDisclosures: S. de Souza, None; A. Bassett, None; R. Williams, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.

