Back
Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0150–0180) Muscle Biology, Myositis and Myopathies Poster I
0150: Growth and Differentiation Factor 15, an Emerging Biomarker of Mitochondrial Dysfunction- Associated Myopathies: Implications for Juvenile Dermatomyositis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BD
Bhargavi Duvvuri, PhD
University of Washington
Seattle, WA, United States
Abstract Poster Presenter(s)
Bhargavi Duvvuri1, Lauren Pachman2, Gabrielle Morgan3, Payton Hermanson4, TING WANG4 and Christian Lood4, 1University of Washington, Seattle, WA, 2Northwestern's Feinberg School of Medicine. Ann and Robert H. Lurie Children's Hospital of Chicago; Stanley Manne Children's Research Institute of Chicago, Lake Forest, IL, 3Ann & Robert H. Lurie Children's Hospital of Chicago and Northwestern University Feinberg School of Medicine, Chicago, IL, 4Division of Rheumatology, University of Washington, Seattle, WA
Background/Purpose: Our prior work has demonstrated mitochondrial involvement in JDM including the accumulation of calcified mitochondria in affected muscle tissue, and elevated levels of circulating mitochondrial markers associating with calcinosis. To further explore the role of mitochondrial damage in JDM, we measured the levels of growth and differentiation factor 15 (GDF-15) in plasma of patients with JDM. GDF-15 is a myomitokine; a muscle-derived factor induced in conditions of mitochondrial dysfunction, and was reported to be a specific biomarker for muscle manifesting mitochondrial diseases. Given the specificity of GDF-15 for mitochondrial myopathy, we hypothesized that GDF-15 could be a novel biomarker for implicating the role of mitochondrial dysfunction in JDM pathobiology as compared to more commonly used markers of myopathy including creatine kinase.
Methods: GDF-15 levels were measured in banked plasma samples by quantitative sandwich ELISA (Human GDF-15 DuoSet ELISA, R&D Systems, Inc.) according to manufacturer's instructions. The neopterin level was measured by a competitive enzyme-linked immunosorbent assay (ALPCO diagnostics kit) in the clinical immunology lab at the Ann & Robert H. Lurie Children's Hospital of Chicago. Group comparisons and correlations are performed using non-parametric Mann-Whitney test, and Spearman correlation tests, respectively. Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported.
Results: Median GDF-15 levels in patients with JDM (n=57) were significantly higher than age-matched healthy controls (n=20) (653.5 pg/mL vs. 313.7 pg/mL; p=0.007, Figure 1). GDF-15 levels were particularly elevated in patients with select myositis-specific antibodies, including p155/p140 (549.0 pg/mL vs. 313.7 pg/mL, p=0.01), and MJ (796.5 pg/mL vs. 313.7 pg/mL, p=0.008). Further analysis revealed a correlation of GDF-15 levels in JDM patients with creatine kinase levels, a traditional marker of muscle damage (r=0.41, p=0.007). Of note, a stronger correlation was observed between GDF-15 and total disease activity score (DAS) than observed for CK and DAS (CK and DAS: r=0.35, p=0.008 vs. GDF-15 and DAS: r=0.71, p=0.006), suggesting that GDF-15 may be a more specific marker of myopathy in patients with mitochondrial abnormalities. Consistently, the correlation between GDF-15 levels and different measures of disease activity including DAS skin score (r=0.56, p=0.006), DAS muscle score (r=0.66, p=0.006), Nailfold End Row Loops (r=-0.33, p=0.01), and The Childhood Myositis Assessment Score, CMAS (r=-0.60, p=0.006), remained statistically significant. It was noteworthy to confirm a strong correlation between GDF-15 and markers of inflammation, tissue localization and muscle damage that include neopterin (r=0.70, p=0.006) and low NK cell count (r=-0.38, p=0.008) in children with JDM.
Conclusion: GDF-15 is a potentially useful indicator of both mitochondrial myopathy and chronic inflammation in JDM.
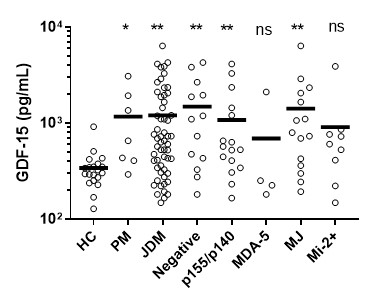 Figure 1. Elevated levels of circulating GDF-15 in patients with JDM. Comparison of GDF-15 levels in the plasma of patients with JDM, disease controls, patients with myositis-specific antibodies and age-matched healthy controls. Group comparisons are performed using non-parametric Mann-Whitney test, and Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported in the main text.
Figure 1. Elevated levels of circulating GDF-15 in patients with JDM. Comparison of GDF-15 levels in the plasma of patients with JDM, disease controls, patients with myositis-specific antibodies and age-matched healthy controls. Group comparisons are performed using non-parametric Mann-Whitney test, and Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported in the main text.
Disclosures: B. Duvvuri, None; L. Pachman, None; G. Morgan, None; P. Hermanson, None; T. WANG, None; C. Lood, Eli Lilly, Gilead Sciences, Pfizer, Bristol-Myers Squibb(BMS), Redd Pharma, Horizon Diagnostic, Exagen Diagnostic.
Background/Purpose: Our prior work has demonstrated mitochondrial involvement in JDM including the accumulation of calcified mitochondria in affected muscle tissue, and elevated levels of circulating mitochondrial markers associating with calcinosis. To further explore the role of mitochondrial damage in JDM, we measured the levels of growth and differentiation factor 15 (GDF-15) in plasma of patients with JDM. GDF-15 is a myomitokine; a muscle-derived factor induced in conditions of mitochondrial dysfunction, and was reported to be a specific biomarker for muscle manifesting mitochondrial diseases. Given the specificity of GDF-15 for mitochondrial myopathy, we hypothesized that GDF-15 could be a novel biomarker for implicating the role of mitochondrial dysfunction in JDM pathobiology as compared to more commonly used markers of myopathy including creatine kinase.
Methods: GDF-15 levels were measured in banked plasma samples by quantitative sandwich ELISA (Human GDF-15 DuoSet ELISA, R&D Systems, Inc.) according to manufacturer's instructions. The neopterin level was measured by a competitive enzyme-linked immunosorbent assay (ALPCO diagnostics kit) in the clinical immunology lab at the Ann & Robert H. Lurie Children's Hospital of Chicago. Group comparisons and correlations are performed using non-parametric Mann-Whitney test, and Spearman correlation tests, respectively. Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported.
Results: Median GDF-15 levels in patients with JDM (n=57) were significantly higher than age-matched healthy controls (n=20) (653.5 pg/mL vs. 313.7 pg/mL; p=0.007, Figure 1). GDF-15 levels were particularly elevated in patients with select myositis-specific antibodies, including p155/p140 (549.0 pg/mL vs. 313.7 pg/mL, p=0.01), and MJ (796.5 pg/mL vs. 313.7 pg/mL, p=0.008). Further analysis revealed a correlation of GDF-15 levels in JDM patients with creatine kinase levels, a traditional marker of muscle damage (r=0.41, p=0.007). Of note, a stronger correlation was observed between GDF-15 and total disease activity score (DAS) than observed for CK and DAS (CK and DAS: r=0.35, p=0.008 vs. GDF-15 and DAS: r=0.71, p=0.006), suggesting that GDF-15 may be a more specific marker of myopathy in patients with mitochondrial abnormalities. Consistently, the correlation between GDF-15 levels and different measures of disease activity including DAS skin score (r=0.56, p=0.006), DAS muscle score (r=0.66, p=0.006), Nailfold End Row Loops (r=-0.33, p=0.01), and The Childhood Myositis Assessment Score, CMAS (r=-0.60, p=0.006), remained statistically significant. It was noteworthy to confirm a strong correlation between GDF-15 and markers of inflammation, tissue localization and muscle damage that include neopterin (r=0.70, p=0.006) and low NK cell count (r=-0.38, p=0.008) in children with JDM.
Conclusion: GDF-15 is a potentially useful indicator of both mitochondrial myopathy and chronic inflammation in JDM.
 Figure 1. Elevated levels of circulating GDF-15 in patients with JDM. Comparison of GDF-15 levels in the plasma of patients with JDM, disease controls, patients with myositis-specific antibodies and age-matched healthy controls. Group comparisons are performed using non-parametric Mann-Whitney test, and Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported in the main text.
Figure 1. Elevated levels of circulating GDF-15 in patients with JDM. Comparison of GDF-15 levels in the plasma of patients with JDM, disease controls, patients with myositis-specific antibodies and age-matched healthy controls. Group comparisons are performed using non-parametric Mann-Whitney test, and Holm (-Bonferroni) correction is used for multiple comparisons and corrected p-values are reported in the main text. Disclosures: B. Duvvuri, None; L. Pachman, None; G. Morgan, None; P. Hermanson, None; T. WANG, None; C. Lood, Eli Lilly, Gilead Sciences, Pfizer, Bristol-Myers Squibb(BMS), Redd Pharma, Horizon Diagnostic, Exagen Diagnostic.

