Back
Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0150–0180) Muscle Biology, Myositis and Myopathies Poster I
0170: Minimal Clinically Important Difference in Myositis Response Criteria
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- KS
Khushboo Singh, MD
UPMC McKeesport
Pittsburgh, PA, United States
Abstract Poster Presenter(s)
Khushboo Singh1, Sara Faghihi Kashani2, Tissa Bijoy George3, Siamak Moghadam-Kia4, Dana Ascherman5, Rohit Aggarwal6 and Chester Oddis5, 1UPMC McKeesport, Pittsburgh, PA, 2Stanford University, Stanford, CA, 3UPMC McKeesport, Monroeville, PA, 4University of Pittsburgh Medical Center, Pittsburgh, PA, 5University of Pittsburgh, Pittsburgh, PA, 6Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA
Background/Purpose: ACR/EULAR myositis response criteria were developed to assess the patient's disease activity, response to therapy, and long-term outcome as a standardized measure to be used in clinical and therapeutic trials. The criteria consist of six core set measures which are then combined in a weighted manner to calculate a Total Improvement Score (TIS) to assess minimal, moderate, and major improvement in myositis patients.
Minimal Clinically Important Difference (MCID) of TIS, which is the smallest magnitude of change in a score that is clinically significant for patients and clinicians, has not been evaluated in myositis patients. We aimed to determine the MCID in adult-onset Polymyositis (PM) and Dermatomyositis (DM) patients across two clinical trials and one observational study.
Methods: Adult PM and DM patients from the (Rituximab in Myositis (RIM) trial (N = 148), Tocilizumab in Myositis (TIM) trial (N = 36), and an observational study of physical activity monitors and patient reported outcome (PAMPRO) (N = 50) were included. All six core set measures (extra-muscular, patient and MD global disease activity, MMT, HAQ Disability Index (HAQ-DI), and CK levels) were measured at baseline and 24-weeks, and TIS was assessed at 24-weeks. HAQ-DI as well as physician and patient impression of change were used as anchors to calculate score difference between the two time-points and to determine if they had significant correlation with TIS. The HAQ-DI was categorized into two groups: change in HAQ-DI< 0.22 or ≥ 0.22 based on prior rheumatologic studies of HAQ-DI and change in MCID. Physician impression of change was also categorized into two groups: improved (markedly/moderately/slightly improved) and not-improved (unchanged, slightly/moderately/markedly worse). MCID was calculated by anchor based and distributive methods using effect size and a linear regression model. We calculated the weighted average of MCID of TIS based on physician impression of change and HAQ-DI using the results of the three cohorts.
Results: A total of 216 patients with adult-onset PM and DM were identified for analysis. Patient impression of change did not correlate with TIS, and therefore MCID was not calculated for this anchor. The correlation coefficient between TIS and physician impression of change and HAQ-change of 0.22 across the three different studies are reported in Table 1.
The TIS MCID using HAQ-DI change of 0.22 as the anchor was -21.53, -16.14, -38.64 based on effect size, and 22.14, 12.52, 37.38 based on linear regression in the RIM, TIM and PAMPRO studies, respectively. The TIS MCID using physician impression of change was -22.16, -27.47, -47.75 based on effect size, and 22.44, 27.14, 37.44 based on linear regression in the RIM, TIM and PAMPRO studies, respectively. The weighted average of TIS MCID based on physician impression of change was 26.60 and based on HAQ-DI change of 0.22 was 23.48.
Conclusion: Physician impression of change and HAQ-DI change analysis suggested that the MCID of TIS should be 26.60 and 23.48, respectively. These values are higher than the threshold set by physician consensus (TIS ≥ 20). Other independent and patient outcome-based measures did not contribute to these models.
.jpg) Table 1: Spearman correlation coefficient of physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22 with TIS
Table 1: Spearman correlation coefficient of physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22 with TIS
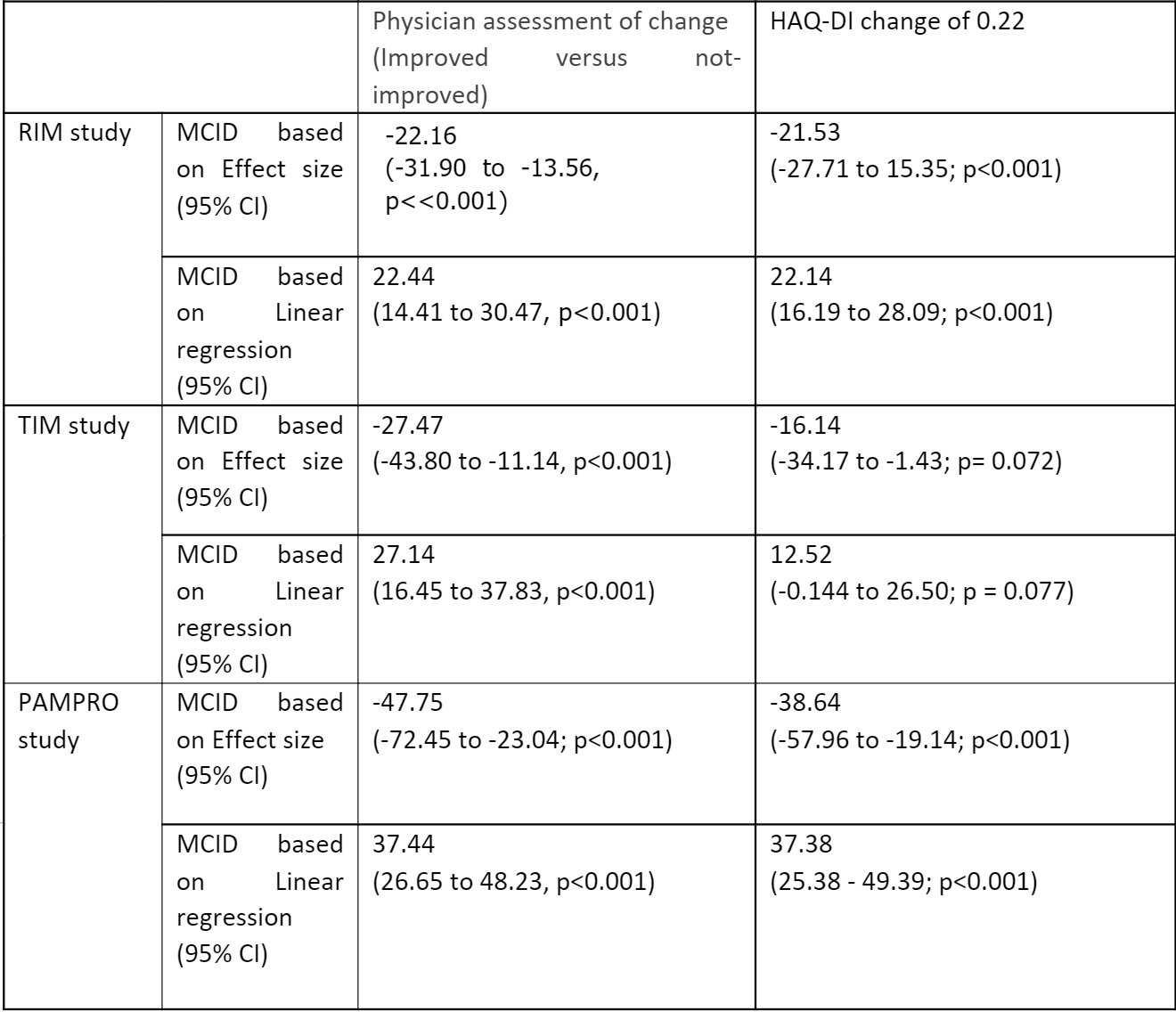 Table 2: MCID of TIS across three different cohorts based on physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22
Table 2: MCID of TIS across three different cohorts based on physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22
Disclosures: K. Singh, None; S. Faghihi Kashani, None; T. Bijoy George, None; S. Moghadam-Kia, None; D. Ascherman, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; C. Oddis, None.
Background/Purpose: ACR/EULAR myositis response criteria were developed to assess the patient's disease activity, response to therapy, and long-term outcome as a standardized measure to be used in clinical and therapeutic trials. The criteria consist of six core set measures which are then combined in a weighted manner to calculate a Total Improvement Score (TIS) to assess minimal, moderate, and major improvement in myositis patients.
Minimal Clinically Important Difference (MCID) of TIS, which is the smallest magnitude of change in a score that is clinically significant for patients and clinicians, has not been evaluated in myositis patients. We aimed to determine the MCID in adult-onset Polymyositis (PM) and Dermatomyositis (DM) patients across two clinical trials and one observational study.
Methods: Adult PM and DM patients from the (Rituximab in Myositis (RIM) trial (N = 148), Tocilizumab in Myositis (TIM) trial (N = 36), and an observational study of physical activity monitors and patient reported outcome (PAMPRO) (N = 50) were included. All six core set measures (extra-muscular, patient and MD global disease activity, MMT, HAQ Disability Index (HAQ-DI), and CK levels) were measured at baseline and 24-weeks, and TIS was assessed at 24-weeks. HAQ-DI as well as physician and patient impression of change were used as anchors to calculate score difference between the two time-points and to determine if they had significant correlation with TIS. The HAQ-DI was categorized into two groups: change in HAQ-DI< 0.22 or ≥ 0.22 based on prior rheumatologic studies of HAQ-DI and change in MCID. Physician impression of change was also categorized into two groups: improved (markedly/moderately/slightly improved) and not-improved (unchanged, slightly/moderately/markedly worse). MCID was calculated by anchor based and distributive methods using effect size and a linear regression model. We calculated the weighted average of MCID of TIS based on physician impression of change and HAQ-DI using the results of the three cohorts.
Results: A total of 216 patients with adult-onset PM and DM were identified for analysis. Patient impression of change did not correlate with TIS, and therefore MCID was not calculated for this anchor. The correlation coefficient between TIS and physician impression of change and HAQ-change of 0.22 across the three different studies are reported in Table 1.
The TIS MCID using HAQ-DI change of 0.22 as the anchor was -21.53, -16.14, -38.64 based on effect size, and 22.14, 12.52, 37.38 based on linear regression in the RIM, TIM and PAMPRO studies, respectively. The TIS MCID using physician impression of change was -22.16, -27.47, -47.75 based on effect size, and 22.44, 27.14, 37.44 based on linear regression in the RIM, TIM and PAMPRO studies, respectively. The weighted average of TIS MCID based on physician impression of change was 26.60 and based on HAQ-DI change of 0.22 was 23.48.
Conclusion: Physician impression of change and HAQ-DI change analysis suggested that the MCID of TIS should be 26.60 and 23.48, respectively. These values are higher than the threshold set by physician consensus (TIS ≥ 20). Other independent and patient outcome-based measures did not contribute to these models.
.jpg) Table 1: Spearman correlation coefficient of physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22 with TIS
Table 1: Spearman correlation coefficient of physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22 with TIS  Table 2: MCID of TIS across three different cohorts based on physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22
Table 2: MCID of TIS across three different cohorts based on physician assessment of change (improved versus not-improved) and HAQ-DI change of 0.22 Disclosures: K. Singh, None; S. Faghihi Kashani, None; T. Bijoy George, None; S. Moghadam-Kia, None; D. Ascherman, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; C. Oddis, None.

