Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0272: Long-Term Efficacy of Baricitinib in Patients with Rheumatoid Arthritis with Inadequate Response to Bdmards: Results from RA-BEYOND up to 6.9-Years of Treatment
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
- DA
Daniel Aletaha, MD, MS, MBA
Medical University Vienna
Wien, Austria
Abstract Poster Presenter(s)
Roberto Caporali1, Daniel Aletaha2, Raimon Sanmarti3, Tsutomu Takeuchi4, DAOJUN MO5, Ewa Haladyj6, Liliana Zaremba-Pechmann7 and Peter Taylor8, 1Dept. of Clinical Sciences & Community Health, Research Center for Adult and Pediatric Rheumatic Diseases, Università degli Studi di Milano, Montu Beccaria, Italy, 2Medical University Vienna, Wien, Austria, 3Hospital Clínic de Barcelona, Barcelona, Spain, 4Keio University and Saitama Medical University, Tokyo, Japan, 5Eli Lilly and Company, Carmel, IN, 6Eli Lilly and Company, Warszawa, Poland, 7HaaPACS GmbH, Schriesheim, Germany, 8University of Oxford, Oxford, United Kingdom
Background/Purpose: Baricitinib (BARI), an oral selective Janus kinase 1/2 inhibitor, is approved for treatment of adults with moderately-to-severely active rheumatoid arthritis (RA). BARI demonstrated efficacy in patients (pts) with RA who have inadequate response to biologic disease-modifying antirheumatic drugs (bDMARD-IR) in a 24-week (wk) phase 3 study, RA-BEACON.1 BARI efficacy was evaluated up to 3 years (yrs) of treatment in a long-term extension (LTE) study, RA-BEYOND.2 This study discloses long-term efficacy of BARI 4 mg and 2 mg in bDMARD-IR pts in the completed study RA-BEYOND.
Methods: In RA-BEACON, pts were randomized 1:1:1 to BARI 4 mg, 2 mg, or PBO; pts with no response could be rescued after wk 16. Completers to wk 24 could enter with BARI 4 or 2mg RA-BEYOND for up to 360 wks (6.9 yrs). LTE data were analysed by treatment assigned at baseline in RA-BEACON as observed up to time of stepdown (if applicable), study discontinuation, or study completion, whichever occurred earlier. Efficacy response rates (RR) were assessed as proportions of pts with observed data up to wk 360 for low-disease activity (LDA) (SDAI ≤ 11, DAS28-hsCRP ≤ 3.2, CDAI ≤ 10), remission (REM) (SDAI ≤ 3.3, DAS28-hsCRP < 2.6, CDAI ≤ 2.8, Boolean), and physical functioning (HAQ-DI ≤ 0.5). No formal statistical comparisons were conducted.
Results: 156, 152, and 140 pts entered the LTE (4 mg, 2 mg, and PBO, respectively). Pts in BARI 4 and 2 mg arms had higher LDA and REM RR vs PBO at LTE entry (wk 24) (Table 1). PBO-treated pts achieved comparable RR to pts in the BARI 4 mg arm by wk 48 (24 wks after switch to BARI 4 mg) and up to wk 360. Of pts enrolled to RA-BEYOND, approx. 50% in BARI 4 mg, 65% in 2 mg and 61% in PBO remained active at wk 156; 17%, 26% and 26% at wk 360, respectively. SDAI LDA RR were 47%/70% and 61%/74% for pts treated with BARI 4 mg and 2 mg, at wk 156 (yr 3)/ 360 (yr 6.9), respectively; SDAI REM RR were 15%/26% and 26%/26% for BARI 4 mg and 2 mg, at wk 156/360, respectively (Table 1). SDAI and CDAI had comparable RR. DAS-28CRP LDA RR were similar to SDAI and CDAI, while REM RR were about twice those of SDAI and CDAI. HAQ-DI ≤ 0.5 RR was 15%/26% (BARI 4 mg), 21%/15% (BARI 2mg), and 9%/3% (PBO) at 3/6.9 yrs.
Conclusion: In observed data, BARI maintained efficacy and normative physical function bDMARD-IR population up to 6.9 yrs (360 wks).
References:
1. Genovese MC et al. N Engl J Med. 2016; 374:1243-52
2. Wells AF et al. Rheumatol Ther. 2021; 8:987–1001
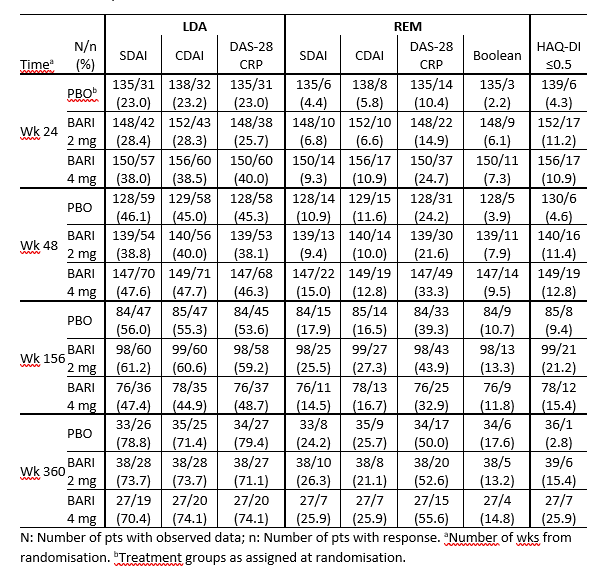 Table 1: Efficacy outcomes in RA-BEYOND
Table 1: Efficacy outcomes in RA-BEYOND
Disclosures: R. Caporali, AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Eli Lilly and Company, Fresenius Kabi, Galapagos NV, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sandoz, UCB Pharma; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; R. Sanmarti, Thermo Fisher Scientific; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; D. MO, Eli Lilly and Company; E. Haladyj, Eli Lilly and Company; L. Zaremba-Pechmann, None; P. Taylor, Biogen, Celltrion, Eli Lilly, Fresenius Kabi, Gilead, GlaxoSmithKlein(GSK), Janssen, Nordic Pharma, Pfizer, Roche, Sanofi, UCB, Galapagos, Abbvie.
Background/Purpose: Baricitinib (BARI), an oral selective Janus kinase 1/2 inhibitor, is approved for treatment of adults with moderately-to-severely active rheumatoid arthritis (RA). BARI demonstrated efficacy in patients (pts) with RA who have inadequate response to biologic disease-modifying antirheumatic drugs (bDMARD-IR) in a 24-week (wk) phase 3 study, RA-BEACON.1 BARI efficacy was evaluated up to 3 years (yrs) of treatment in a long-term extension (LTE) study, RA-BEYOND.2 This study discloses long-term efficacy of BARI 4 mg and 2 mg in bDMARD-IR pts in the completed study RA-BEYOND.
Methods: In RA-BEACON, pts were randomized 1:1:1 to BARI 4 mg, 2 mg, or PBO; pts with no response could be rescued after wk 16. Completers to wk 24 could enter with BARI 4 or 2mg RA-BEYOND for up to 360 wks (6.9 yrs). LTE data were analysed by treatment assigned at baseline in RA-BEACON as observed up to time of stepdown (if applicable), study discontinuation, or study completion, whichever occurred earlier. Efficacy response rates (RR) were assessed as proportions of pts with observed data up to wk 360 for low-disease activity (LDA) (SDAI ≤ 11, DAS28-hsCRP ≤ 3.2, CDAI ≤ 10), remission (REM) (SDAI ≤ 3.3, DAS28-hsCRP < 2.6, CDAI ≤ 2.8, Boolean), and physical functioning (HAQ-DI ≤ 0.5). No formal statistical comparisons were conducted.
Results: 156, 152, and 140 pts entered the LTE (4 mg, 2 mg, and PBO, respectively). Pts in BARI 4 and 2 mg arms had higher LDA and REM RR vs PBO at LTE entry (wk 24) (Table 1). PBO-treated pts achieved comparable RR to pts in the BARI 4 mg arm by wk 48 (24 wks after switch to BARI 4 mg) and up to wk 360. Of pts enrolled to RA-BEYOND, approx. 50% in BARI 4 mg, 65% in 2 mg and 61% in PBO remained active at wk 156; 17%, 26% and 26% at wk 360, respectively. SDAI LDA RR were 47%/70% and 61%/74% for pts treated with BARI 4 mg and 2 mg, at wk 156 (yr 3)/ 360 (yr 6.9), respectively; SDAI REM RR were 15%/26% and 26%/26% for BARI 4 mg and 2 mg, at wk 156/360, respectively (Table 1). SDAI and CDAI had comparable RR. DAS-28CRP LDA RR were similar to SDAI and CDAI, while REM RR were about twice those of SDAI and CDAI. HAQ-DI ≤ 0.5 RR was 15%/26% (BARI 4 mg), 21%/15% (BARI 2mg), and 9%/3% (PBO) at 3/6.9 yrs.
Conclusion: In observed data, BARI maintained efficacy and normative physical function bDMARD-IR population up to 6.9 yrs (360 wks).
References:
1. Genovese MC et al. N Engl J Med. 2016; 374:1243-52
2. Wells AF et al. Rheumatol Ther. 2021; 8:987–1001
 Table 1: Efficacy outcomes in RA-BEYOND
Table 1: Efficacy outcomes in RA-BEYONDDisclosures: R. Caporali, AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Eli Lilly and Company, Fresenius Kabi, Galapagos NV, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sandoz, UCB Pharma; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; R. Sanmarti, Thermo Fisher Scientific; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; D. MO, Eli Lilly and Company; E. Haladyj, Eli Lilly and Company; L. Zaremba-Pechmann, None; P. Taylor, Biogen, Celltrion, Eli Lilly, Fresenius Kabi, Gilead, GlaxoSmithKlein(GSK), Janssen, Nordic Pharma, Pfizer, Roche, Sanofi, UCB, Galapagos, Abbvie.

