Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0276: Baseline Characteristics of and Early Outcomes in the First 200 Patients with RA Treated with Filgotinib in a Prospective Observational Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JG
James Galloway, MBBS
King's College London
London, United Kingdom
Abstract Poster Presenter(s)
James Galloway1, Karen Bevers2, Patrick Verschueren3, Roberto Felice Caporali4, Susana Romero Yuste5, Jérôme Avouac6, Emilia Gvozdenovic7, Kristina Harris7, Monia Zignani7 and Gerd Burmester8, 1King's College London, London, United Kingdom, 2Sint Maartenskliniek, Nijmegen, Netherlands, 3University Hospital Leuven, Leuven, Belgium, 4University of Milan, Milano, Italy, 5Complexo Hospitalario Universitario, Pontevedra, Spain, 6University of Paris, Paris, France, 7Galapagos NV, Mechelen, Belgium, 8Charité University Medicine Berlin, Berlin, Germany
Background/Purpose: Filgotinib (FIL) is a preferential Janus kinase 1 inhibitor, approved in Europe for the treatment of RA and ulcerative colitis. A phase 4 European study of FIL (FILOSOPHY; NCT04871919) in patients with RA is ongoing. We report baseline characteristics and 1-month efficacy data from the first 200 patients enrolled.
Methods: The prospective, observational study will enroll approximately 1500 patients aged ≥18 years with moderate to severe active RA, who are prescribed FIL for the first time and in accordance with the product label in general practice. We report baseline demographics, disease characteristics, comorbidities, prior treatments, reasons for starting FIL, and mean change from baseline (CFB) in severity of pain (measured using a 10-cm visual analog scale [VAS]) and Functional Assessment of Chronic Illness Therapy (FACIT)-fatigue score (range 0–52) via electronic PRO data collection at Week 1 and Month 1, and mean CFB in Clinical Disease Activity Index (CDAI; range 0–76) score at Month 1. The proportion of patients with clinically meaningful CFB in FACIT-fatigue (increase of ≥4) and VAS pain (reduction of ≥10) from Week 1 to Month 1 was reported. Patients included in the analysis were enrolled between May 2021 and February 2022.
Results: Table 1). Approximately 40% of treated patients had comorbidities, the most common being hypertension (28.3%), dyslipidemia (9.1%), and type 2 diabetes mellitus (5.3%). Baseline disease characteristics and details of previous treatments are included in Table 1. FIL was started due to inadequate response (43.3%), loss of response (40.6%), or intolerance (non-allergic) to previous treatment (5.9%), or for other reasons (10.2%). Of treated patients, 167 (89.3%) received FIL 200 mg and 20 (10.7%) received FIL 100 mg (study drug information was pending for 19 patients). FIL monotherapy was taken by 55.6% of patients (19.3% with glucocorticoids), while 37.4% received FIL in combination with MTX (13.4% with glucocorticoids). Mean (SD) and median (range) daily dose of oral glucocorticoids (prednisone or equivalent) at baseline was 7.5 (6.0) mg and 5.0 (1.0-30.0) mg. At Week 1 and Month 1, respectively, mean (SD) CFB in FACIT-fatigue score was 3.7 (6.9) and 6.8 (8.6), and mean (SD) CFB in VAS pain score was -11.9 (18.0) and -22.2 (27.8). At Month 1, mean (SD) CFB in CDAI score was -13.7 (17.1) (Table 2). The Figure shows the proportions of patients with clinically relevant CFB in FACIT-fatigue and VAS pain scores.
Conclusion: These are the first international real-world data to be reported from a cohort of patients with RA treated with FIL. At baseline, patients generally had moderate disease activity. Initial interim data indicate rapid improvements in disease measures with FIL treatment (given as combination therapy or monotherapy, with/without glucocorticoids), which could be observed as early as Week 1 for VAS pain and FACIT-fatigue scores. Long-term follow-up is needed to further evaluate effectiveness and safety.
.jpg) Table 1. Baseline characteristics, laboratory measures, and prior and current treatment
Table 1. Baseline characteristics, laboratory measures, and prior and current treatment
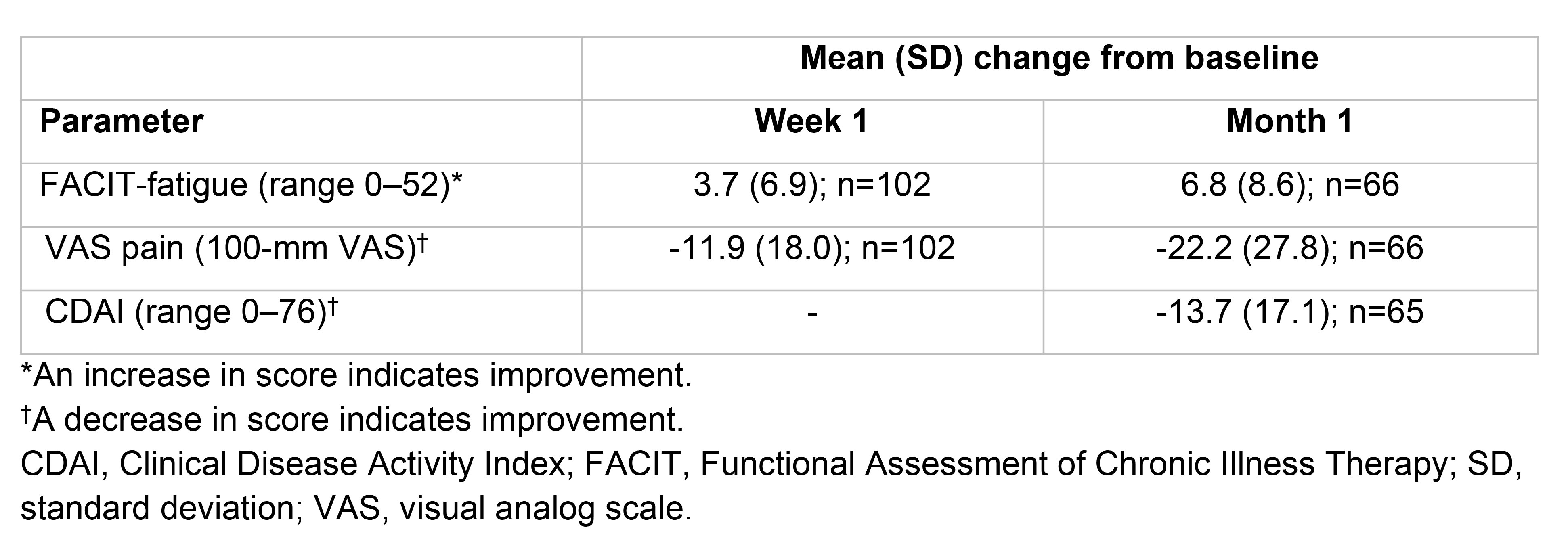 Table 2. Mean change from baseline in FACIT-fatigue, VAS pain, and CDAI scores at Week 1 and Month 1
Table 2. Mean change from baseline in FACIT-fatigue, VAS pain, and CDAI scores at Week 1 and Month 1
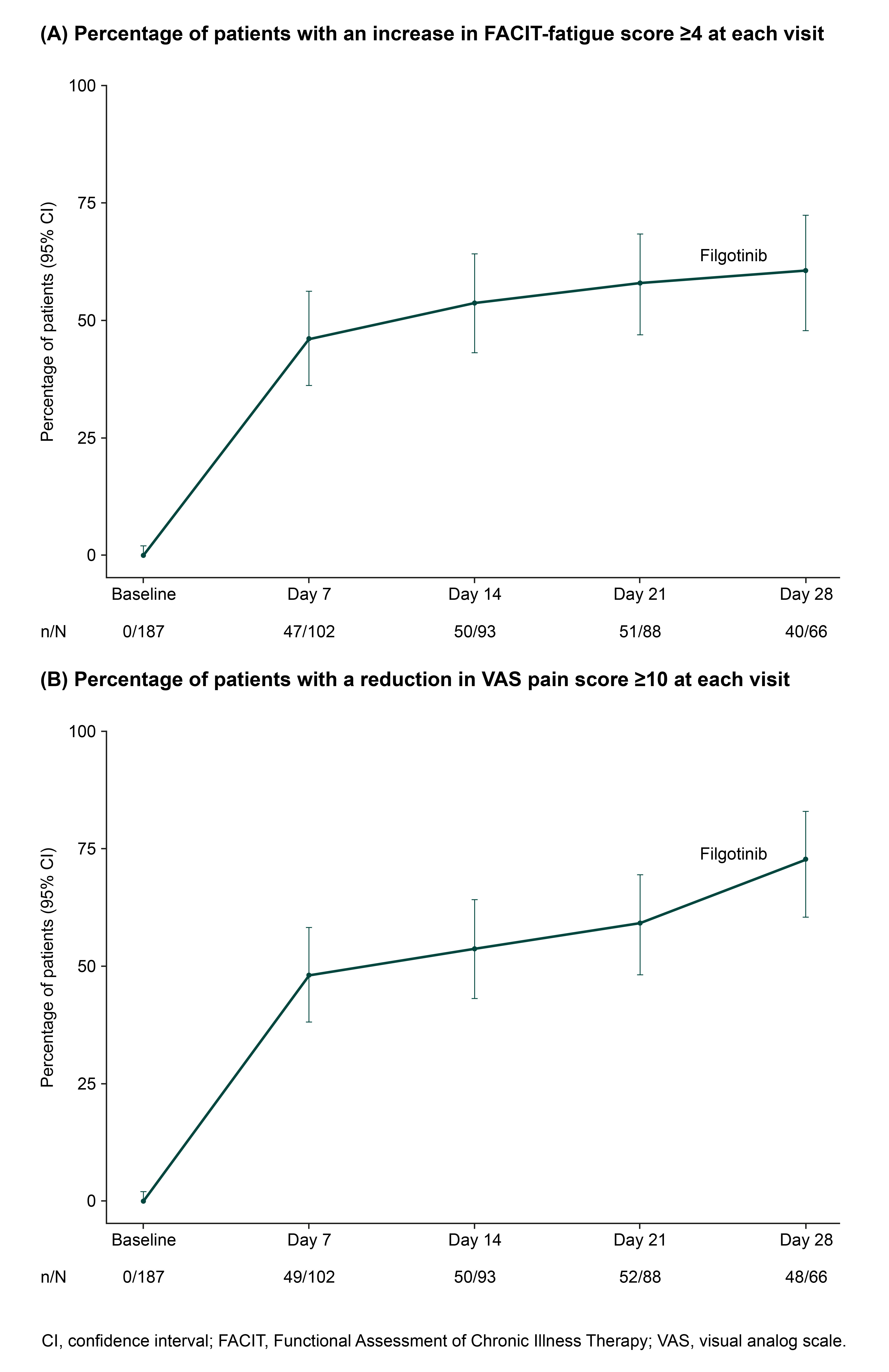 Figure. Proportion of patients with clinically meaningful improvement from baseline in (A) FACIT-fatigue and (B) VAS pain scores
Figure. Proportion of patients with clinically meaningful improvement from baseline in (A) FACIT-fatigue and (B) VAS pain scores
Disclosures: J. Galloway, AbbVie, AstraZeneca, Biogen, Celgene, Galapagos, Gilead, Janssen, Medicago, Lilly, Novartis, Novavax, Pfizer, Roche, UCB; K. Bevers, None; P. Verschueren, AbbVie, Celltrion, Eli Lilly, Galapagos, Gilead, Nordic Pharma, Pfizer, Roularta, Sidekick Health; R. Caporali, AbbVie, Celltrion, Fresenius-Kabi, Galapagos, Janssen, Pfizer, Roche, UCB, Eli Lilly, Gilead, Sanofi; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; E. Gvozdenovic, Galapagos; K. Harris, Galapagos; M. Zignani, Galapagos; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc..
Background/Purpose: Filgotinib (FIL) is a preferential Janus kinase 1 inhibitor, approved in Europe for the treatment of RA and ulcerative colitis. A phase 4 European study of FIL (FILOSOPHY; NCT04871919) in patients with RA is ongoing. We report baseline characteristics and 1-month efficacy data from the first 200 patients enrolled.
Methods: The prospective, observational study will enroll approximately 1500 patients aged ≥18 years with moderate to severe active RA, who are prescribed FIL for the first time and in accordance with the product label in general practice. We report baseline demographics, disease characteristics, comorbidities, prior treatments, reasons for starting FIL, and mean change from baseline (CFB) in severity of pain (measured using a 10-cm visual analog scale [VAS]) and Functional Assessment of Chronic Illness Therapy (FACIT)-fatigue score (range 0–52) via electronic PRO data collection at Week 1 and Month 1, and mean CFB in Clinical Disease Activity Index (CDAI; range 0–76) score at Month 1. The proportion of patients with clinically meaningful CFB in FACIT-fatigue (increase of ≥4) and VAS pain (reduction of ≥10) from Week 1 to Month 1 was reported. Patients included in the analysis were enrolled between May 2021 and February 2022.
Results: Table 1). Approximately 40% of treated patients had comorbidities, the most common being hypertension (28.3%), dyslipidemia (9.1%), and type 2 diabetes mellitus (5.3%). Baseline disease characteristics and details of previous treatments are included in Table 1. FIL was started due to inadequate response (43.3%), loss of response (40.6%), or intolerance (non-allergic) to previous treatment (5.9%), or for other reasons (10.2%). Of treated patients, 167 (89.3%) received FIL 200 mg and 20 (10.7%) received FIL 100 mg (study drug information was pending for 19 patients). FIL monotherapy was taken by 55.6% of patients (19.3% with glucocorticoids), while 37.4% received FIL in combination with MTX (13.4% with glucocorticoids). Mean (SD) and median (range) daily dose of oral glucocorticoids (prednisone or equivalent) at baseline was 7.5 (6.0) mg and 5.0 (1.0-30.0) mg. At Week 1 and Month 1, respectively, mean (SD) CFB in FACIT-fatigue score was 3.7 (6.9) and 6.8 (8.6), and mean (SD) CFB in VAS pain score was -11.9 (18.0) and -22.2 (27.8). At Month 1, mean (SD) CFB in CDAI score was -13.7 (17.1) (Table 2). The Figure shows the proportions of patients with clinically relevant CFB in FACIT-fatigue and VAS pain scores.
Conclusion: These are the first international real-world data to be reported from a cohort of patients with RA treated with FIL. At baseline, patients generally had moderate disease activity. Initial interim data indicate rapid improvements in disease measures with FIL treatment (given as combination therapy or monotherapy, with/without glucocorticoids), which could be observed as early as Week 1 for VAS pain and FACIT-fatigue scores. Long-term follow-up is needed to further evaluate effectiveness and safety.
.jpg) Table 1. Baseline characteristics, laboratory measures, and prior and current treatment
Table 1. Baseline characteristics, laboratory measures, and prior and current treatment Table 2. Mean change from baseline in FACIT-fatigue, VAS pain, and CDAI scores at Week 1 and Month 1
Table 2. Mean change from baseline in FACIT-fatigue, VAS pain, and CDAI scores at Week 1 and Month 1 Figure. Proportion of patients with clinically meaningful improvement from baseline in (A) FACIT-fatigue and (B) VAS pain scores
Figure. Proportion of patients with clinically meaningful improvement from baseline in (A) FACIT-fatigue and (B) VAS pain scoresDisclosures: J. Galloway, AbbVie, AstraZeneca, Biogen, Celgene, Galapagos, Gilead, Janssen, Medicago, Lilly, Novartis, Novavax, Pfizer, Roche, UCB; K. Bevers, None; P. Verschueren, AbbVie, Celltrion, Eli Lilly, Galapagos, Gilead, Nordic Pharma, Pfizer, Roularta, Sidekick Health; R. Caporali, AbbVie, Celltrion, Fresenius-Kabi, Galapagos, Janssen, Pfizer, Roche, UCB, Eli Lilly, Gilead, Sanofi; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; E. Gvozdenovic, Galapagos; K. Harris, Galapagos; M. Zignani, Galapagos; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc..

