Back
Poster Session A
Vasculitis
Session: (0432–0457) Vasculitis – ANCA-Associated Poster I: Epidemiology, Outcomes, and Classification
0437: Outcome Measures in Patients with Neurologic Involvement in ANCA-associated Vasculitis: Data from Multicenter Longitudinal Observational Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Rula Hajj-Ali, MD
Cleveland Clinic
Hunting Valley, OH, United States
Abstract Poster Presenter(s)
Rula Hajj-Ali1, Carol Langford2, Chao Zhang2, Leonard Calabrese2, David Cuthbertson3, Nader Khalidi4, Curry Koening5, Carol McAlear6, Paul Monach7, Larry Moreland8, Christian Pagnoux9, Ulrich Specks10, Antoine Sreih11, Kenneth J. Warrington10 and Peter Merkel12, 1Cleveland Clinic, Hunting Valley, OH, 2Cleveland Clinic, Cleveland, OH, 3University of South Florida, Tampa, FL, 4McMaster University, Hamilton, ON, Canada, 5University of Texas Dell Medical School, Austin, TX, 6Division of Rheumatology, University of Pennsylvania, Philadelphia, PA, USA, Philadelphia, PA, 7VA Boston Healthcare System, Boston, MA, 8University of Colorado, Denver, CO, 9Mount Sinai Hospital, Toronto, ON, Canada, 10Mayo Clinic, Rochester, MN, 11Bristol Myers Squibb, Philadelphia, PA, 12University of Pennsylvania, Philadelphia, PA
Background/Purpose: Neurologic involvement (NI) in ANCA-associated vasculitis (AAV) is common, occurring in approximately one-third of patients, and leads to significant and often chronic functional limitations and bothersome symptoms. There are limited data on health-related quality of life (HRQOL) in patients with NI in AAV. This project aimed to report on patient-reported outcome measures in patients with AAV with or without NI.
Methods: Data for this analysis is derived from patients ≥18 years old with AAV [granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), or eosinophilic granulomatosis with polyangiitis (EGPA)] participating in a multicenter longitudinal observational study from 2006-2021 through the Vasculitis Clinical Research Consortium. HRQOL was assessed with the SF-36 health survey that includes physical and mental component summary scores (PCS and MCS, respectively), PROMIS domains (Fatigue, Pain Interference, Physical Function, Applied Cognitive Abilities, Social Isolation and Anxiety) acquired by computer adaptive testing, and patient global assessment (PGA). Patient subsets were compared according to presence or absence of NI.
Results: Data from 1368 patients with GPA or MPA were available. Complete data were available on 1361/1368 patients for SF36, 1359/1368 for PGA, 978/1368 for PROMIS.
Based on the SF-36, compared to patients without NI, patients with NI have lower scores in Physical Functioning (p < 0.001), Role Limitations (Physical) (p = 0.005), Bodily Pain (p < 0.001), and PCS (p < 0.001) (Figure 1). PROMIS measurements indicated that compared with patients without NI, patients with NI had higher Pain Interference scores (p = 0.033) and lower Physical Function scores (p = 0.047) (Figure 2). Patients with NI reported more vasculitis disease severity as measured by PGA (p = 0.006).
Conclusion: NI in patients with AAV has a negative impact on multiple domains of illness and health-related quality of life. Patients with NI have worse physical functioning, limitations in physical role, and more pain compared to those without NI. These data are informative for clinicians and patients with AAV and should raise the awareness of the impairment in patient with NI in AAV.
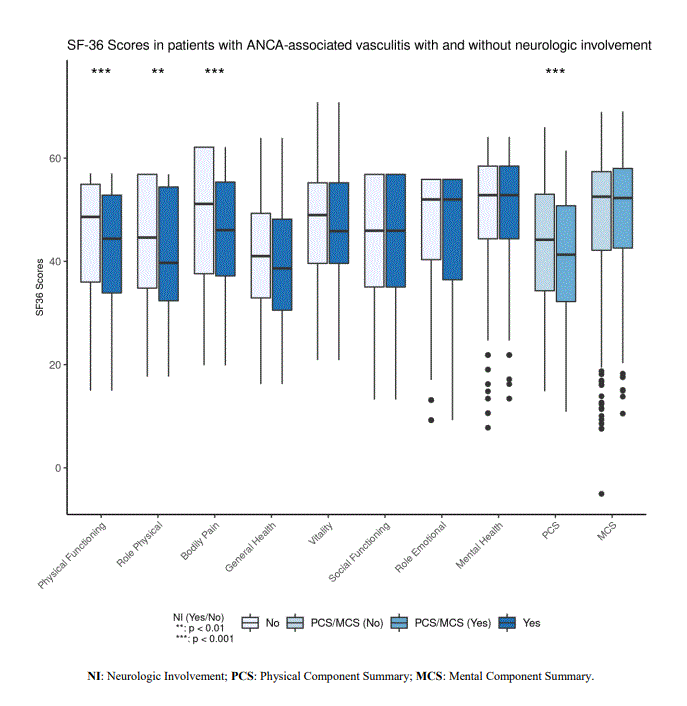 Figure 1- SF 36 scores in patients with ANCA-associated vasculitis with and without neurologic involvement
Figure 1- SF 36 scores in patients with ANCA-associated vasculitis with and without neurologic involvement
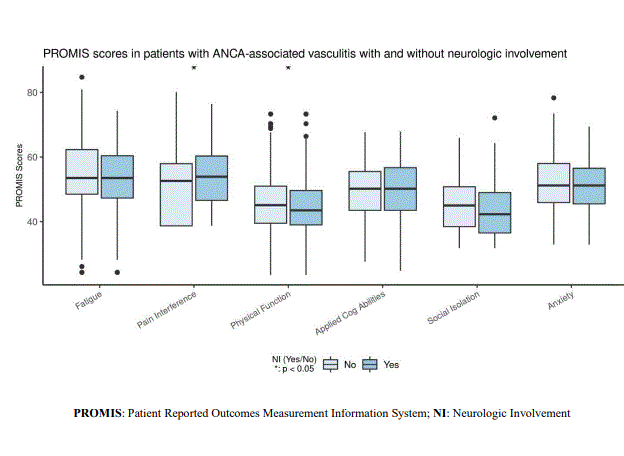 Figure 2- PROMIS scores in patients with ANCA-associated vasculitis with and without neurologic involvement
Figure 2- PROMIS scores in patients with ANCA-associated vasculitis with and without neurologic involvement
Disclosures: R. Hajj-Ali, uptodate; C. Langford, None; C. Zhang, None; L. Calabrese, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Genentech, Janssen, UCB, Sanofi, Regeneron, Galvani, GlaxoSmithKlein(GSK), AstraZeneca, Chemocentryx; D. Cuthbertson, None; N. Khalidi, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Roche, Sanofi, AbbVie/Abbott, Katsaka Medical, Otsuka; C. Koening, chemocentryx; C. McAlear, None; P. Monach, Chemocentryx, Kiniksa, BMS/Celgene, Gilead; L. Moreland, None; C. Pagnoux, otsuka, AstraZeneca, pfizer; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; A. Sreih, Bristol Myers Squibb; K. Warrington, Eli Lilly, GlaxoSmithKlein(GSK), Kiniksa, Chemocentryx; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.
Background/Purpose: Neurologic involvement (NI) in ANCA-associated vasculitis (AAV) is common, occurring in approximately one-third of patients, and leads to significant and often chronic functional limitations and bothersome symptoms. There are limited data on health-related quality of life (HRQOL) in patients with NI in AAV. This project aimed to report on patient-reported outcome measures in patients with AAV with or without NI.
Methods: Data for this analysis is derived from patients ≥18 years old with AAV [granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), or eosinophilic granulomatosis with polyangiitis (EGPA)] participating in a multicenter longitudinal observational study from 2006-2021 through the Vasculitis Clinical Research Consortium. HRQOL was assessed with the SF-36 health survey that includes physical and mental component summary scores (PCS and MCS, respectively), PROMIS domains (Fatigue, Pain Interference, Physical Function, Applied Cognitive Abilities, Social Isolation and Anxiety) acquired by computer adaptive testing, and patient global assessment (PGA). Patient subsets were compared according to presence or absence of NI.
Results: Data from 1368 patients with GPA or MPA were available. Complete data were available on 1361/1368 patients for SF36, 1359/1368 for PGA, 978/1368 for PROMIS.
Based on the SF-36, compared to patients without NI, patients with NI have lower scores in Physical Functioning (p < 0.001), Role Limitations (Physical) (p = 0.005), Bodily Pain (p < 0.001), and PCS (p < 0.001) (Figure 1). PROMIS measurements indicated that compared with patients without NI, patients with NI had higher Pain Interference scores (p = 0.033) and lower Physical Function scores (p = 0.047) (Figure 2). Patients with NI reported more vasculitis disease severity as measured by PGA (p = 0.006).
Conclusion: NI in patients with AAV has a negative impact on multiple domains of illness and health-related quality of life. Patients with NI have worse physical functioning, limitations in physical role, and more pain compared to those without NI. These data are informative for clinicians and patients with AAV and should raise the awareness of the impairment in patient with NI in AAV.
 Figure 1- SF 36 scores in patients with ANCA-associated vasculitis with and without neurologic involvement
Figure 1- SF 36 scores in patients with ANCA-associated vasculitis with and without neurologic involvement  Figure 2- PROMIS scores in patients with ANCA-associated vasculitis with and without neurologic involvement
Figure 2- PROMIS scores in patients with ANCA-associated vasculitis with and without neurologic involvement Disclosures: R. Hajj-Ali, uptodate; C. Langford, None; C. Zhang, None; L. Calabrese, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Genentech, Janssen, UCB, Sanofi, Regeneron, Galvani, GlaxoSmithKlein(GSK), AstraZeneca, Chemocentryx; D. Cuthbertson, None; N. Khalidi, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Roche, Sanofi, AbbVie/Abbott, Katsaka Medical, Otsuka; C. Koening, chemocentryx; C. McAlear, None; P. Monach, Chemocentryx, Kiniksa, BMS/Celgene, Gilead; L. Moreland, None; C. Pagnoux, otsuka, AstraZeneca, pfizer; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; A. Sreih, Bristol Myers Squibb; K. Warrington, Eli Lilly, GlaxoSmithKlein(GSK), Kiniksa, Chemocentryx; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.

