Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0408: Long-Term Clinical Outcomes of Certolizumab Pegol Treatment in Patients with Active Non‑Radiographic Axial Spondyloarthritis Stratified by Baseline MRI and C-Reactive Protein Status
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Philip Robinson, PhD, FRACP, MBChB
University of Queensland
Herston, Queensland, Australia
Abstract Poster Presenter(s)
Philip C. Robinson1, Walter P Maksymowych2, Lianne Gensler3, Martin Rudwaleit4, Bengt Hoepken5, Lars Bauer5, Thomas Kumke5, Mindy Kim6 and Atul Deodhar7, 1University of Queensland School of Clinical Medicine, Brisbane, Australia, 2Department of Medicine, University of Alberta, Edmonton, AB, Canada, 3Department of Medicine, Division of Rheumatology, University of California San Francisco, San Francisco, CA, 4University of Bielefeld, Klinikum Bielefeld, Bielefeld; Germany Klinikum Bielefeld and Charité Berlin, Germany, and Gent University, Gent, Belgium, 5UCB Pharma, Monheim am Rhein, Germany, 6UCB Pharma, Smyrna, GA, 7Oregon Health & Science University, Portland, OR, USA, Portland, OR
Background/Purpose: Certolizumab pegol (CZP) has demonstrated clinical efficacy in patients (pts) with active non-radiographic axial spondyloarthritis (nr-axSpA) and objective signs of inflammation (OSI) during the 52-week (wk) placebo (PBO)-controlled period and the 104 wk open-label (OL) safety follow-up extension (SFE) of the C-axSpAnd study.1 There is, however, a paucity of data on the long-term efficacy of biologics in nr-axSpA according to pts' baseline MRI and CRP status. This post hoc analysis aimed to evaluate whether pts' baseline MRI and CRP status impacted long-term (3-year [yr]) clinical responses to CZP.
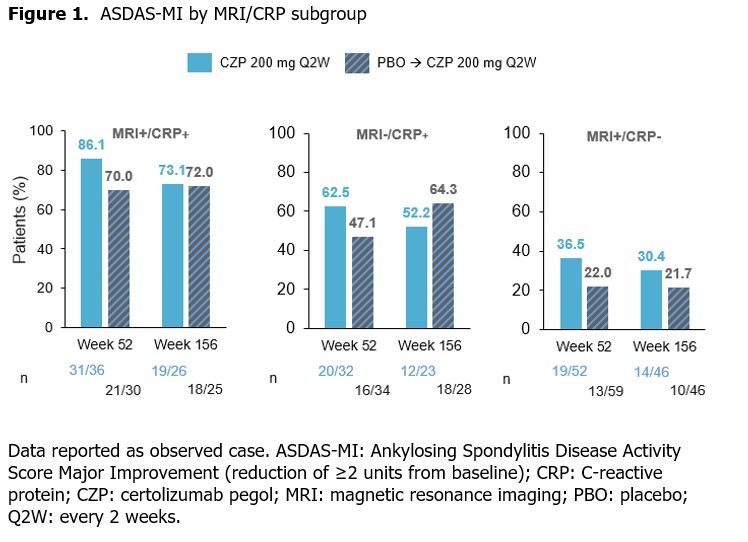
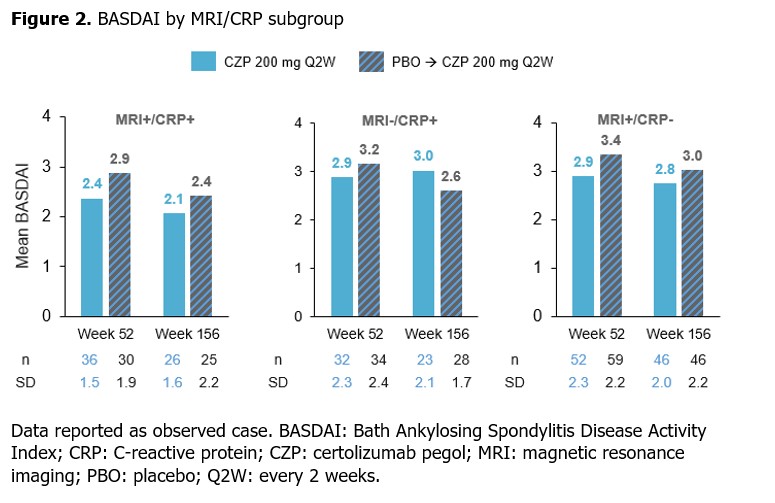
Methods: C-axSpAnd (NCT02552212) was a 3-yr, phase 3, multicenter study. Adults (N=317) with active nr-axSpA, defined by fulfilling the Assessment of SpondyloArthritis international Society (ASAS) classification criteria and OSI (CRP ≥10 mg/L [CRP+] and/or evidence of sacroiliitis on MRI [MRI+]),2 were randomized 1:1 to PBO or CZP (400 mg at Wks 0, 2, 4 then 200 mg every 2 wks [Q2W]) for 52 wks.3 Those enrolled into the SFE received OL CZP (200 mg Q2W) for an additional 104 wks.Ankylosing Spondylitis Disease Activity Score (ASDAS), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), ASDAS major improvement (ASDAS-MI; C-axSpAnd primary outcome) and ASAS 40% response (ASAS40) were assessed at Wks 52 and 156 according to prespecified subgroups based on MRI/CRP status (MRI+/CRP+, MRI−/CRP+, MRI+/CRP−). Data are reported as observed case.&
Results: 243/317 (76.7%) pts entered the SFE, 120 initially randomized to CZP (36 MRI+/CRP+, 32 MRI−/CRP+ and 52 MRI+/CRP−) and 123 from the initial PBO group (30 MRI+/CRP+, 34 MRI−/CRP+ and 59 MRI+/CRP−). 206/243 (84.8%) completed the SFE; 102/120 (85.0%) from the group initially randomized to CZP, 104/123 (84.6%) from the initial PBO group.
Among CZP-randomized pts, mean ASDAS was similar at Wks 52 and 156; the percentage achieving ASDAS-MI was lower at Wk 156 compared with Wk 52 across all subgroups (Figure 1). Pts initially randomized to PBO showed improvements in mean ASDAS over time; ASDAS-MI achievement was sustained. The proportion of patients achieving ASDAS-MI was numerically highest in the MRI+/CRP+ subgroup, and lowest in the MRI+/CRP− subgroup.
Similar results were shown for BASDAI, with mean scores for CZP-randomized pts sustained from Wk 52 to Wk 156 across all subgroups (Figure 2). Mean BASDAI decreased from Wk 52 to Wk 156 in pts initially randomized to PBO, at which point the values aligned with those reported for the CZP-randomized group.
In CZP-randomized pts, ASAS40 responses were sustained at Wk 156 compared with Wk 52. An increased percentage of pts achieved ASAS40 in all MRI/CRP subgroups initially randomized to PBO at Wk 156 compared with Wk 52 (Figure 3).
Conclusion: In pts with nr-axSpA and OSI, long-term clinical outcomes achieved after 1 yr were generally sustained at 3 yrs across MRI+/CRP+, MRI−/CRP+ and MRI+/CRP− subgroups; ASDAS-MI was numerically highest in the MRI+/CRP+ subgroup.References: 1. van der Heijde D. Arthritis Rheumatol 2021;73(suppl 10); 2. Lambert RG. Ann Rheum Dis 2016;75(11):1958–63; 3. Deodhar A. Arthritis Rheumatol 2019;71(7):1101–11.
.jpg)
Disclosures: P. Robinson, AbbVie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche, UCB Pharma, Bristol-Myers Squibb (BMS); W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; B. Hoepken, UCB Pharma; L. Bauer, UCB Pharma; T. Kumke, UCB Pharma; M. Kim, UCB Pharma; A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake.
Background/Purpose: Certolizumab pegol (CZP) has demonstrated clinical efficacy in patients (pts) with active non-radiographic axial spondyloarthritis (nr-axSpA) and objective signs of inflammation (OSI) during the 52-week (wk) placebo (PBO)-controlled period and the 104 wk open-label (OL) safety follow-up extension (SFE) of the C-axSpAnd study.1 There is, however, a paucity of data on the long-term efficacy of biologics in nr-axSpA according to pts' baseline MRI and CRP status. This post hoc analysis aimed to evaluate whether pts' baseline MRI and CRP status impacted long-term (3-year [yr]) clinical responses to CZP.
Methods: C-axSpAnd (NCT02552212) was a 3-yr, phase 3, multicenter study. Adults (N=317) with active nr-axSpA, defined by fulfilling the Assessment of SpondyloArthritis international Society (ASAS) classification criteria and OSI (CRP ≥10 mg/L [CRP+] and/or evidence of sacroiliitis on MRI [MRI+]),2 were randomized 1:1 to PBO or CZP (400 mg at Wks 0, 2, 4 then 200 mg every 2 wks [Q2W]) for 52 wks.3 Those enrolled into the SFE received OL CZP (200 mg Q2W) for an additional 104 wks.Ankylosing Spondylitis Disease Activity Score (ASDAS), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), ASDAS major improvement (ASDAS-MI; C-axSpAnd primary outcome) and ASAS 40% response (ASAS40) were assessed at Wks 52 and 156 according to prespecified subgroups based on MRI/CRP status (MRI+/CRP+, MRI−/CRP+, MRI+/CRP−). Data are reported as observed case.&
Results: 243/317 (76.7%) pts entered the SFE, 120 initially randomized to CZP (36 MRI+/CRP+, 32 MRI−/CRP+ and 52 MRI+/CRP−) and 123 from the initial PBO group (30 MRI+/CRP+, 34 MRI−/CRP+ and 59 MRI+/CRP−). 206/243 (84.8%) completed the SFE; 102/120 (85.0%) from the group initially randomized to CZP, 104/123 (84.6%) from the initial PBO group.
Among CZP-randomized pts, mean ASDAS was similar at Wks 52 and 156; the percentage achieving ASDAS-MI was lower at Wk 156 compared with Wk 52 across all subgroups (Figure 1). Pts initially randomized to PBO showed improvements in mean ASDAS over time; ASDAS-MI achievement was sustained. The proportion of patients achieving ASDAS-MI was numerically highest in the MRI+/CRP+ subgroup, and lowest in the MRI+/CRP− subgroup.
Similar results were shown for BASDAI, with mean scores for CZP-randomized pts sustained from Wk 52 to Wk 156 across all subgroups (Figure 2). Mean BASDAI decreased from Wk 52 to Wk 156 in pts initially randomized to PBO, at which point the values aligned with those reported for the CZP-randomized group.
In CZP-randomized pts, ASAS40 responses were sustained at Wk 156 compared with Wk 52. An increased percentage of pts achieved ASAS40 in all MRI/CRP subgroups initially randomized to PBO at Wk 156 compared with Wk 52 (Figure 3).
Conclusion: In pts with nr-axSpA and OSI, long-term clinical outcomes achieved after 1 yr were generally sustained at 3 yrs across MRI+/CRP+, MRI−/CRP+ and MRI+/CRP− subgroups; ASDAS-MI was numerically highest in the MRI+/CRP+ subgroup.References: 1. van der Heijde D. Arthritis Rheumatol 2021;73(suppl 10); 2. Lambert RG. Ann Rheum Dis 2016;75(11):1958–63; 3. Deodhar A. Arthritis Rheumatol 2019;71(7):1101–11.


.jpg)
Disclosures: P. Robinson, AbbVie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche, UCB Pharma, Bristol-Myers Squibb (BMS); W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; B. Hoepken, UCB Pharma; L. Bauer, UCB Pharma; T. Kumke, UCB Pharma; M. Kim, UCB Pharma; A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake.

