Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0348: Significant Improvement in Cutaneous Lupus Erythematosus with or Without Systemic Lupus Erythematosus with Belimumab Use – a Systematic Review and Meta-Analysis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SG
Shivani Garg, MD, MS
University of Madison, School of Medicine and Public Health
Madison, WI, United States
Abstract Poster Presenter(s)
Rachel Kneeland1, Daniel Montes1, Justin endo1, Bridget Shields1, Christie Bartels2 and Shivani Garg3, 1University of Wisconsin, School of Medicine and Public Health, Madison, WI, 2University of Wisconsin School of Medicine and Public Health, Madison, WI, 3University of Madison, School of Medicine and Public Health, Madison, WI
Background/Purpose: Cutaneous lupus erythematosus (CLE) with or without systemic lupus erythematosus (SLE), can be debilitating and cause significant scarring and psychological distress. Belimumab, a monoclonal antibody that inhibits B-cell activation, is one of only four FDA-approved medications for SLE, but less is known on its use in CLE with or without SLE. Moreover, the time to response after starting belimumab in patients with cutaneous disease is unknown, which may lead to premature discontinuation in the absence of early perceivable benefits. Thus, objectives of this meta-analysis were to examine: a) the efficacy of belimumab; b) time to response after starting belimumab in patients with CLE with or without SLE.
Methods: A comprehensive search was performed using MeSH headings and keywords (e.g., lupus, cutaneous, or discoid, and belimumab) in Medline, Embase, CINAHL and Web of Science. We included observational and interventional belimumab studies that examined clinical response in patients with CLE with or without SLE. Newcastle Ottawa Scale and Cochrane Collaboration Risk Assessment tools were used to rate the quality of observational and interventional studies.
Clinical response at 52 weeks in belimumab users vs. non-users was the primary outcome examined using a random effects modeling (rma) in R. Clinical response was defined as a decrease in the cutaneous domain from a baseline BILAG A or B score, to a BILAG score of B-E. Additionally, we calculated the pooled odds ratio for each consecutive 4-week observation interval to trend clinical response time, and the time to achieve sustained response in CLE with or without SLE after starting belimumab. Finally, we pooled odds of cutaneous flares in belimumab users vs. non-users at 52 weeks. Heterogeneity was assessed using I2.
Results: Among 745 manually reviewed abstracts, 13 met inclusion. Only six blinded interventional studies examining the cutaneous response in patients with SLE or CLE to belimumab versus non-users were included in the meta-analysis. The odds of clinical response at 52 weeks in belimumab users were 44% higher compared to non-users (OR 1.44, 95% CI 1.20-1.74, p < 0.001, I2 0%, Fig. 1). Clinical response was first noted after 20 weeks of starting belimumab (OR 1.35, 95% CI 1.01-1.81, p 0.04, I2 0%), with a sustained clinical response thereafter peaking at one year (Fig. 2). Belimumab users had 49% lower risk of severe cutaneous flares compared to non-users (OR 0.51, 95% CI 0.31-0.84, p < 0.001, I2 0%; Fig. 3). We noted that 70% of interventional studies had low risk of bias and no heterogeneity was observed.
After including seven observational studies (all without a control group, poor quality on risk of bias assessment), a similar pooled response estimate of 55% was noted in belimumab users.
Conclusion: Our study estimates a strong cutaneous clinical response to belimumab therapy ranging from 44% to 55%, and ~50% lower flare risk over one year in CLE with or without SLE. These findings support belimumab as a good alternative or adjunct therapy for patients with CLE with or without SLE. Likewise, they can inform patient counseling that it takes ~20 weeks to achieve a significant response. Future studies should examine the comparative efficacy of belimumab across CLE subtypes.
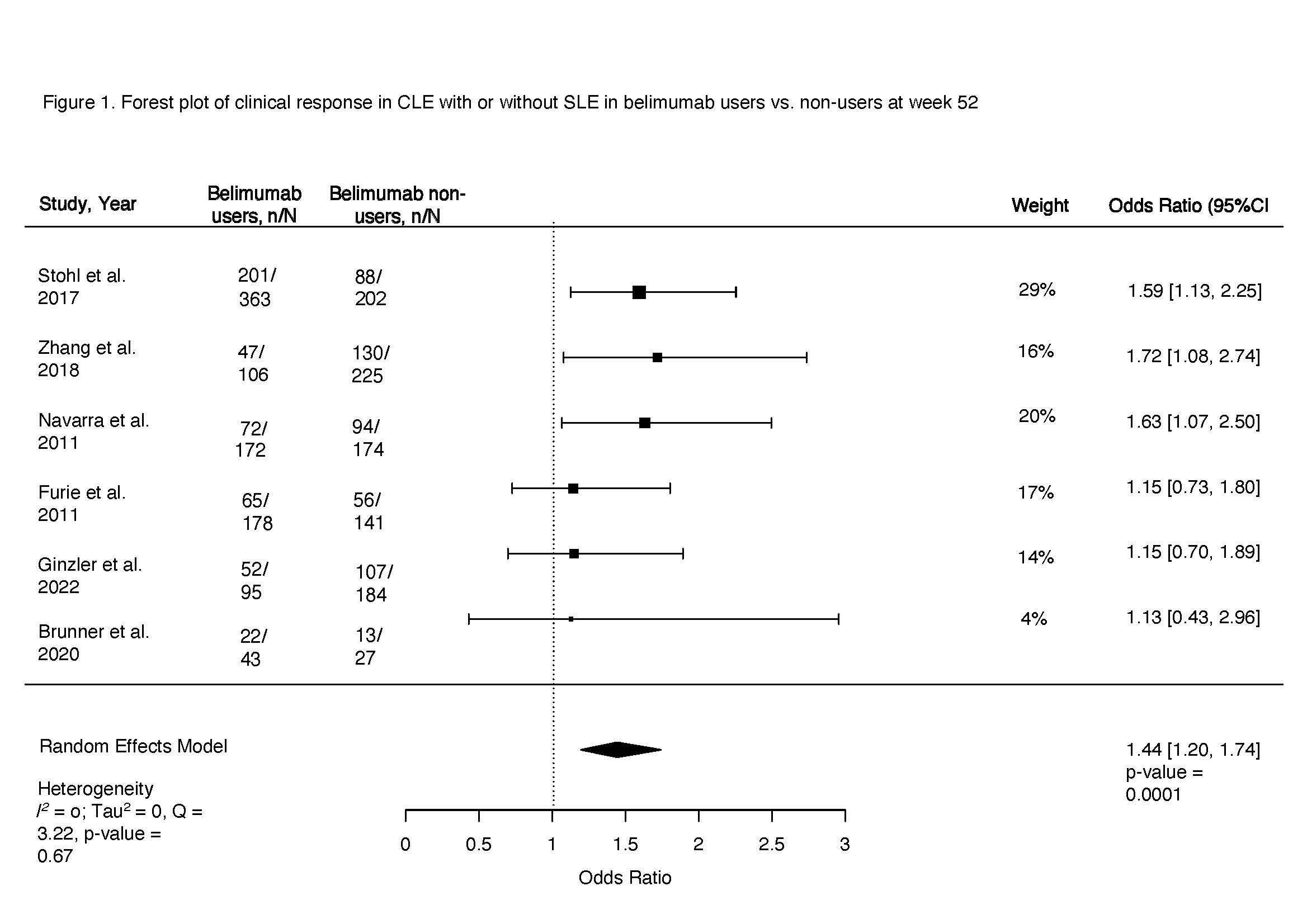 Figure 1. Forest plot of clinical response in CLE with or without SLE in belimumab users vs. non-users at week 52
Figure 1. Forest plot of clinical response in CLE with or without SLE in belimumab users vs. non-users at week 52
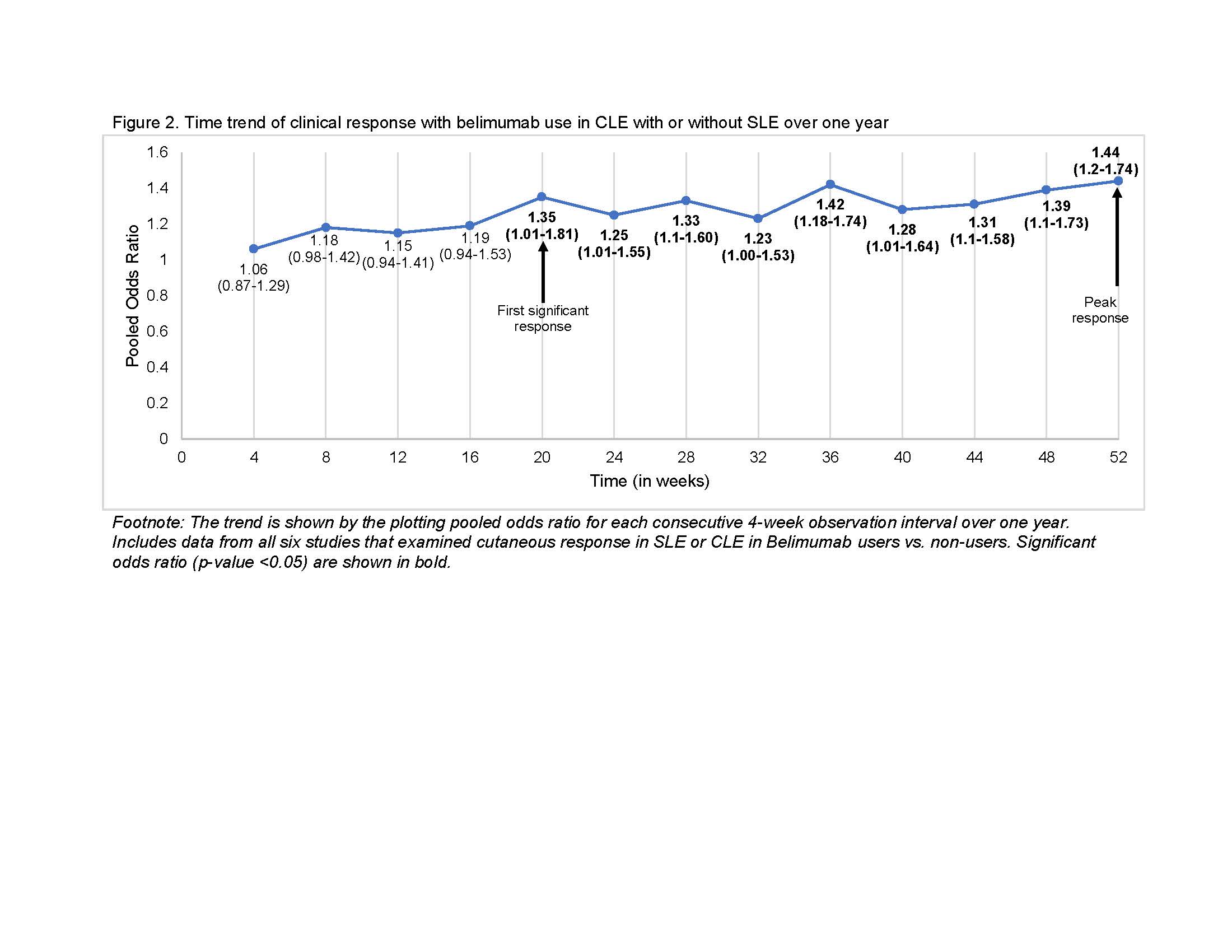 Figure 2. Time trend of clinical response with belimumab use in CLE with or without SLE over one year
Figure 2. Time trend of clinical response with belimumab use in CLE with or without SLE over one year
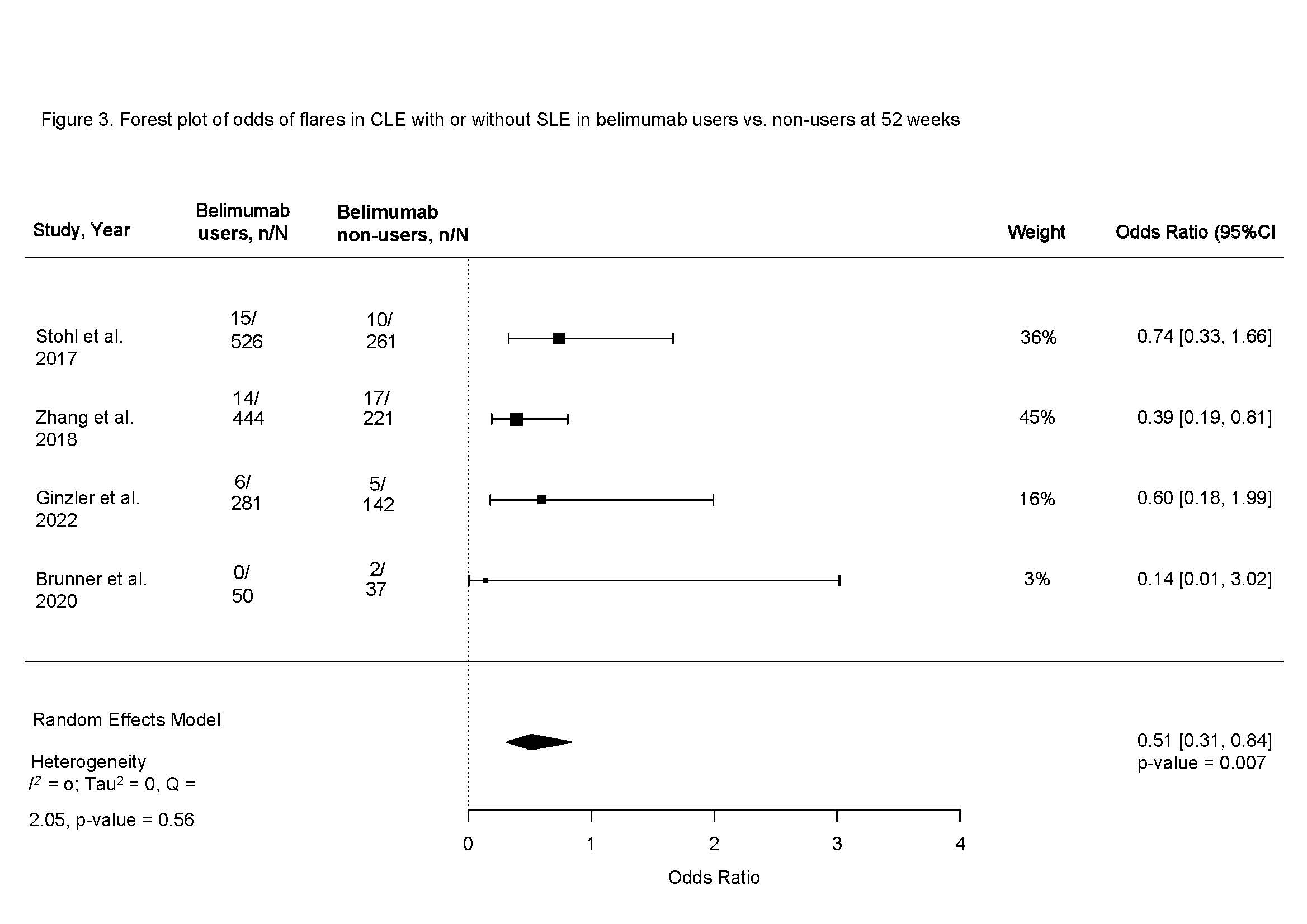 Figure 3. Forest plot of odds of flares in CLE with or without SLE in belimumab users vs. non-users at 52 weeks
Figure 3. Forest plot of odds of flares in CLE with or without SLE in belimumab users vs. non-users at 52 weeks
Disclosures: R. Kneeland, None; D. Montes, None; J. endo, None; B. Shields, None; C. Bartels, Pfizer; S. Garg, None.
Background/Purpose: Cutaneous lupus erythematosus (CLE) with or without systemic lupus erythematosus (SLE), can be debilitating and cause significant scarring and psychological distress. Belimumab, a monoclonal antibody that inhibits B-cell activation, is one of only four FDA-approved medications for SLE, but less is known on its use in CLE with or without SLE. Moreover, the time to response after starting belimumab in patients with cutaneous disease is unknown, which may lead to premature discontinuation in the absence of early perceivable benefits. Thus, objectives of this meta-analysis were to examine: a) the efficacy of belimumab; b) time to response after starting belimumab in patients with CLE with or without SLE.
Methods: A comprehensive search was performed using MeSH headings and keywords (e.g., lupus, cutaneous, or discoid, and belimumab) in Medline, Embase, CINAHL and Web of Science. We included observational and interventional belimumab studies that examined clinical response in patients with CLE with or without SLE. Newcastle Ottawa Scale and Cochrane Collaboration Risk Assessment tools were used to rate the quality of observational and interventional studies.
Clinical response at 52 weeks in belimumab users vs. non-users was the primary outcome examined using a random effects modeling (rma) in R. Clinical response was defined as a decrease in the cutaneous domain from a baseline BILAG A or B score, to a BILAG score of B-E. Additionally, we calculated the pooled odds ratio for each consecutive 4-week observation interval to trend clinical response time, and the time to achieve sustained response in CLE with or without SLE after starting belimumab. Finally, we pooled odds of cutaneous flares in belimumab users vs. non-users at 52 weeks. Heterogeneity was assessed using I2.
Results: Among 745 manually reviewed abstracts, 13 met inclusion. Only six blinded interventional studies examining the cutaneous response in patients with SLE or CLE to belimumab versus non-users were included in the meta-analysis. The odds of clinical response at 52 weeks in belimumab users were 44% higher compared to non-users (OR 1.44, 95% CI 1.20-1.74, p < 0.001, I2 0%, Fig. 1). Clinical response was first noted after 20 weeks of starting belimumab (OR 1.35, 95% CI 1.01-1.81, p 0.04, I2 0%), with a sustained clinical response thereafter peaking at one year (Fig. 2). Belimumab users had 49% lower risk of severe cutaneous flares compared to non-users (OR 0.51, 95% CI 0.31-0.84, p < 0.001, I2 0%; Fig. 3). We noted that 70% of interventional studies had low risk of bias and no heterogeneity was observed.
After including seven observational studies (all without a control group, poor quality on risk of bias assessment), a similar pooled response estimate of 55% was noted in belimumab users.
Conclusion: Our study estimates a strong cutaneous clinical response to belimumab therapy ranging from 44% to 55%, and ~50% lower flare risk over one year in CLE with or without SLE. These findings support belimumab as a good alternative or adjunct therapy for patients with CLE with or without SLE. Likewise, they can inform patient counseling that it takes ~20 weeks to achieve a significant response. Future studies should examine the comparative efficacy of belimumab across CLE subtypes.
 Figure 1. Forest plot of clinical response in CLE with or without SLE in belimumab users vs. non-users at week 52
Figure 1. Forest plot of clinical response in CLE with or without SLE in belimumab users vs. non-users at week 52 Figure 2. Time trend of clinical response with belimumab use in CLE with or without SLE over one year
Figure 2. Time trend of clinical response with belimumab use in CLE with or without SLE over one year Figure 3. Forest plot of odds of flares in CLE with or without SLE in belimumab users vs. non-users at 52 weeks
Figure 3. Forest plot of odds of flares in CLE with or without SLE in belimumab users vs. non-users at 52 weeksDisclosures: R. Kneeland, None; D. Montes, None; J. endo, None; B. Shields, None; C. Bartels, Pfizer; S. Garg, None.

