Back
Poster Session A
Vasculitis
Session: (0458–0497) Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
0464: JAKINIB in Refractory Giant Cell Arteritis. National Multicenter Study of 15 Cases and Literature Review
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- DP
Diana Prieto-Peña, PhD
Hospital Universitario Marqués de Valdecilla
Santander, Spain
Abstract Poster Presenter(s)
Diana Prieto-Peña1, Javier Loricera2, Susana Romero Yuste3, Anne Riveros-Frutos4, F. Javier Narváez5, Eugenio De Miguel6, Valentina Emperiale7, Elena Becerra-Fernández8, Santos Castañeda9, Eztizen Labrador10, Olga Maiz11, Miguel Ángel González-Gay12 and Ricardo Blanco2, 1Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 3Complexo Hospitalario Universitario, Pontevedra, Spain, 4Hospital Universitari Germans Trias i Pujol, Barcelona, Spain, 5Rheumatology Department, Hospital Universitario de Bellvitge, Barcelona, Spain, 6Hospital Universitario La Paz, Madrid, Spain, 7Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, 8Hospital Universitario de Torrevieja, London, United Kingdom, 9Division of Rheumatology, Hospital Universitario de La Princesa, IIS-Princesa, Madrid, Spain, 10Hospital San Pedro, Logroño, Spain, 11Hospital Universitario de Donostia, San Sebastián, Spain, 12Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Background/Purpose: Giant Cell Arteritis (GCA) may be refractory to standard treatment with glucocorticoids and conventional and/or biologic disease-modifying antirheumatic drugs (cDMARDs/bDMARDs). The JAK/STAT signaling pathway is involved in GCA; thus, JAK inhibitors (JAKINIBs) may be useful in this disease. Baricitinib (BARI) seems useful for GCA in a clinical open-label pilot study (Koster MJ, et al. Ann Rheum Dis. 2022;81:861-8). However, most patients had not previously received DMARDs, and none had received tocilizumab the only biologic drug approved in GCA. We assess the effectiveness and safety of JAKINIBs in refractory GCA to cDMARDs and/or bDMARDs.
Methods: National multicenter study of clinical practice and literature review of JAKINIBs-treated GCA. For the literature review, a search of PubMed, Embase and the Cochrane library was conducted from inception to 15 May 2022. A comparative study between the previous pilot study (Koster MJ, et al. Ann Rheum Dis. 2022 Jun;81(6):861-86) and this series of real clinical practice was performed.
Results: We included 15 patients (14 ,93.3% female) with a mean age of 71.4±8.3 years. They were refractory to methotrexate (MTX) (n=12; 80%), IL-6 receptor antagonists (n=12; 80%), abatacept (ABA) (n=3; 25%), and ustekinumab (USTE) (n=1; 7%). The following JAKINIBs were firstly used, Baricitinib (BARI) (n=7) (4 mg/24 h; n=4) (2 mg/24h; n=3), tofacitinib (TOFA) (n= 5) (5 mg/12h), and upadacitinib (UPA) (n=3) (15 mg/24h). Patients of clinical practice had longer disease duration, received DMARDs more commonly and were on a lower baseline dose of prednisone than those in the open label study (Table 1).
After a median follow-up of 3 [1-11] months, clinical improvement was observed in 6 (85.7%), 3 (60%) and 2 (66.7%) with BARI, TOFA and UPA, respectively. A reduction of prednisone dose was achieved as well (Figure). Elevation of liver function tests was observed in one patient with BARI who was switched to UPA maintaining clinical improvement. No other severe adverse events were reported. Another 2 patients were switched to a second JAKINIB: one patient from UPA to BARI due to inefficacy and another one from TOFA to FILGO due to warning in older age and cardiovascular comorbidities, pending clinical response evaluation due to recent change.
In the literature review we found 21 patients with GCA (17 women, 74.2±.1.7 years). BARI was the most used (n=26, 72.2%). Most patients improved with JAKINIBs (Table 2).
Conclusion: JAKINIBs appear to be an effective and safety therapy in refractory GCA to cDMARDs and/or bDMARDs.
.jpg) TABLE 1. Baseline characteristics of 15 patients with refractory GCA of clinical practice and the open label pilot study.
TABLE 1. Baseline characteristics of 15 patients with refractory GCA of clinical practice and the open label pilot study.
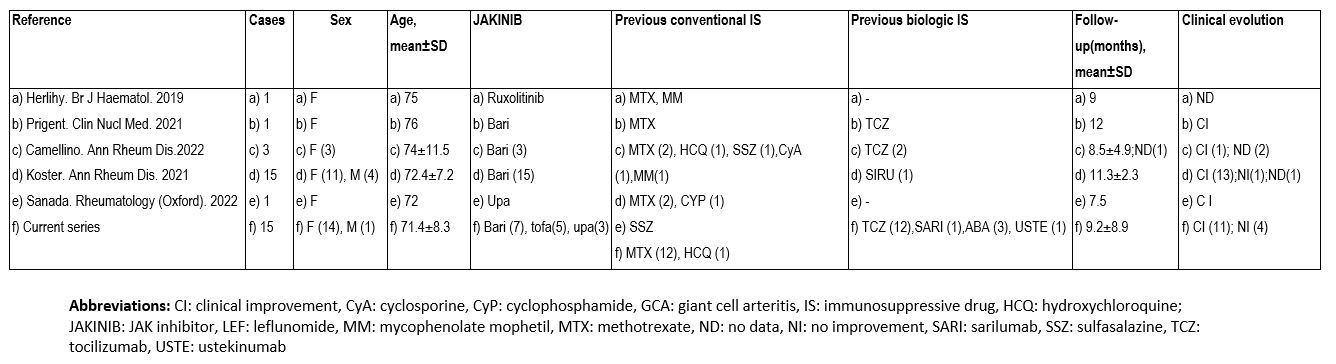 TABLE 2. Present series and literature review of patients with GCA treated with JAKINIBs.
TABLE 2. Present series and literature review of patients with GCA treated with JAKINIBs.
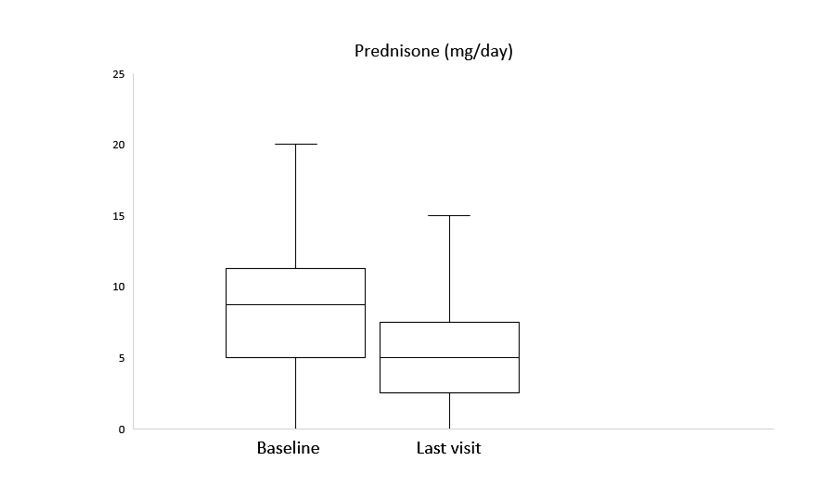 Figure. Reduction of prednisone dose following JAKINIB therapy.
Figure. Reduction of prednisone dose following JAKINIB therapy.
Disclosures: D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; J. Loricera, Novartis, UCB, Celgene, Roche; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; A. Riveros-Frutos, None; F. Narváez, None; E. De Miguel, None; V. Emperiale, None; E. Becerra-Fernández, None; S. Castañeda, Roche; E. Labrador, None; O. Maiz, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Giant Cell Arteritis (GCA) may be refractory to standard treatment with glucocorticoids and conventional and/or biologic disease-modifying antirheumatic drugs (cDMARDs/bDMARDs). The JAK/STAT signaling pathway is involved in GCA; thus, JAK inhibitors (JAKINIBs) may be useful in this disease. Baricitinib (BARI) seems useful for GCA in a clinical open-label pilot study (Koster MJ, et al. Ann Rheum Dis. 2022;81:861-8). However, most patients had not previously received DMARDs, and none had received tocilizumab the only biologic drug approved in GCA. We assess the effectiveness and safety of JAKINIBs in refractory GCA to cDMARDs and/or bDMARDs.
Methods: National multicenter study of clinical practice and literature review of JAKINIBs-treated GCA. For the literature review, a search of PubMed, Embase and the Cochrane library was conducted from inception to 15 May 2022. A comparative study between the previous pilot study (Koster MJ, et al. Ann Rheum Dis. 2022 Jun;81(6):861-86) and this series of real clinical practice was performed.
Results: We included 15 patients (14 ,93.3% female) with a mean age of 71.4±8.3 years. They were refractory to methotrexate (MTX) (n=12; 80%), IL-6 receptor antagonists (n=12; 80%), abatacept (ABA) (n=3; 25%), and ustekinumab (USTE) (n=1; 7%). The following JAKINIBs were firstly used, Baricitinib (BARI) (n=7) (4 mg/24 h; n=4) (2 mg/24h; n=3), tofacitinib (TOFA) (n= 5) (5 mg/12h), and upadacitinib (UPA) (n=3) (15 mg/24h). Patients of clinical practice had longer disease duration, received DMARDs more commonly and were on a lower baseline dose of prednisone than those in the open label study (Table 1).
After a median follow-up of 3 [1-11] months, clinical improvement was observed in 6 (85.7%), 3 (60%) and 2 (66.7%) with BARI, TOFA and UPA, respectively. A reduction of prednisone dose was achieved as well (Figure). Elevation of liver function tests was observed in one patient with BARI who was switched to UPA maintaining clinical improvement. No other severe adverse events were reported. Another 2 patients were switched to a second JAKINIB: one patient from UPA to BARI due to inefficacy and another one from TOFA to FILGO due to warning in older age and cardiovascular comorbidities, pending clinical response evaluation due to recent change.
In the literature review we found 21 patients with GCA (17 women, 74.2±.1.7 years). BARI was the most used (n=26, 72.2%). Most patients improved with JAKINIBs (Table 2).
Conclusion: JAKINIBs appear to be an effective and safety therapy in refractory GCA to cDMARDs and/or bDMARDs.
.jpg) TABLE 1. Baseline characteristics of 15 patients with refractory GCA of clinical practice and the open label pilot study.
TABLE 1. Baseline characteristics of 15 patients with refractory GCA of clinical practice and the open label pilot study. TABLE 2. Present series and literature review of patients with GCA treated with JAKINIBs.
TABLE 2. Present series and literature review of patients with GCA treated with JAKINIBs. Figure. Reduction of prednisone dose following JAKINIB therapy.
Figure. Reduction of prednisone dose following JAKINIB therapy.Disclosures: D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; J. Loricera, Novartis, UCB, Celgene, Roche; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; A. Riveros-Frutos, None; F. Narváez, None; E. De Miguel, None; V. Emperiale, None; E. Becerra-Fernández, None; S. Castañeda, Roche; E. Labrador, None; O. Maiz, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

