Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0248: Monocyte Chemoattractant Protein-1, Vascular Cell Adhesion Protein-1 and Asymmetric Dimethylarginine as Potential Biomarkers of Interstitial Lung Disease Associated with RA
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- VP
Veronica Pulito Cueto, PhD
IDIVAL
Santander, Spain
Abstract Poster Presenter(s)
Veronica Pulito-Cueto1, Sara Remuzgo-Martinez1, Fernanda Genre1, Belén Atienza-Mateo1, Víctor M. Mora-Cuesta1, David Iturbe-Fernández1, Leticia Lera-Gómez1, Diana Prieto-Peña1, Virgi Portilla1, Ricardo Blanco2, Alfonso Corrales1, Oreste Gualillo3, José M. Cifrián1, Raquel Lopez Mejias4 and Miguel Ángel González-Gay5, 1Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 3Hospital Clínico Universitario de Santiago, SERGAS (Servizo Galego de Saude) and IDIS (Instituto de Investigación Sanitaria de Santiago), NEIRID Lab (Neuroendocrine Interactions in Rheumatology and Inflammatory Diseases), Research Laboratory 9, Santiago de Compostela, Spain, 4IDIVAL, Santander, Spain, 5Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Background/Purpose: Interstitial lung disease (ILD) is one of the main comorbidities of patients with RA contributing to an increased mortality risk [1]. The pathogenesis of RA-ILD remains poorly defined, constituting its early diagnosis a challenge for clinicians [1]. The endothelium plays a pivotal role in inflammatory diseases and in the pulmonary vascular regulation contributing to the development of vascular dysfunction and the subsequent onset and progression of ILD [2,3]. Accordingly, the objective of this work was to elucidate the role of monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion protein 1 (VCAM-1) and asymmetric dimethylarginine (ADMA), key molecules in endothelial dysfunction, in the pathological processes of both underlying vascular damage and pulmonary fibrosis in RA-ILD.
Methods: Peripheral venous blood was collected from 21 patients with RA-ILD+ and individuals from 3 comparative groups: 25 patients with RA-ILD-, 21 patients with idiopathic pulmonary fibrosis (IPF) and 21 healthy controls (HC). All the individuals were recruited from the Rheumatology and Pneumology departments of Hospital Universitario Marqués de Valdecilla, Santander, Spain. Serum levels of MCP-1, VCAM-1 and ADMA were measured by enzyme-linked immunosorbent assay.
Results: Patients with RA-ILD+ exhibited significantly higher serum levels of MCP-1, VCAM-1 and ADMA in relation to HC (p< 0.001 in all cases, Figure 1A-C). Likewise, increased levels of MCP-1, VCAM-1 and ADMA were found in patients with RA-ILD+ compared to those with RA-ILD- (p< 0.001, p< 0.001 and p=0.023 respectively, Figure 1A-C). It was also the case when patients with RA-ILD+ were compared with patients with IPF (p< 0.001, p< 0.001 and p=0.001, respectively, Figure 1A-C).
Conclusion: Our study supports a role of MCP-1, VCAM-1 and ADMA in the underlying vasculopathy in RA-ILD+. Interestingly, an increase of serum levels of MCP-1, VCAM-1 and ADMA may help to identify the presence of ILD in patients with RA and to discriminate RA-ILD+ patients from those with IPF.
References: [1] J Clin Med 2019;8(12):2038; [2] Stem Cell Rev Rep 2018;14(2):223-235; [3] Atherosclerosis 2012;224(2):309-317.Personal funds, VP-C and SR-M: RD16/0012/0009(ISCIII-ERDF); FG: RICORS Program (RD21/0002/0025) (ISCIII-EU); RL-M: CP16/00033 (ISCIII-ESF).
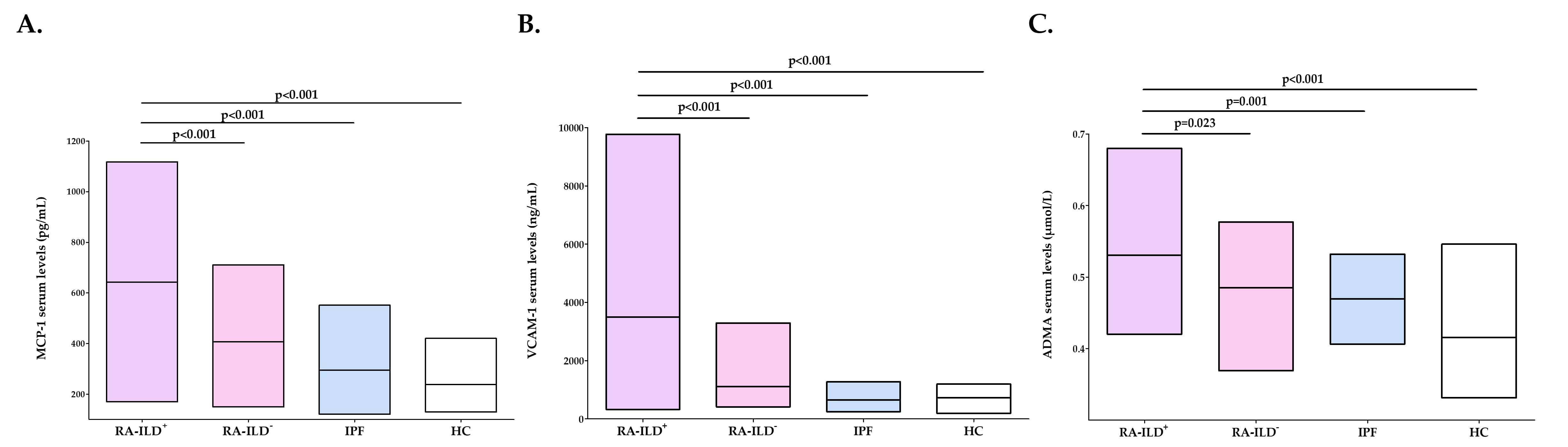 Figure 1. Serum levels of MCP-1 (A), VCAM-1 (B) and ADMA (C) in all the individuals of the study. Significant results are highlighted in bold.
Figure 1. Serum levels of MCP-1 (A), VCAM-1 (B) and ADMA (C) in all the individuals of the study. Significant results are highlighted in bold.
Disclosures: V. Pulito-Cueto, None; S. Remuzgo-Martinez, None; F. Genre, None; B. Atienza-Mateo, AbbVie/Abbott, Roche, Pfizer, Celgene, Novartis, Janssen, UCB, Eli Lilly; V. Mora-Cuesta, None; D. Iturbe-Fernández, None; L. Lera-Gómez, None; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; V. Portilla, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi; A. Corrales, None; O. Gualillo, None; J. Cifrián, None; R. Lopez Mejias, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD.
Background/Purpose: Interstitial lung disease (ILD) is one of the main comorbidities of patients with RA contributing to an increased mortality risk [1]. The pathogenesis of RA-ILD remains poorly defined, constituting its early diagnosis a challenge for clinicians [1]. The endothelium plays a pivotal role in inflammatory diseases and in the pulmonary vascular regulation contributing to the development of vascular dysfunction and the subsequent onset and progression of ILD [2,3]. Accordingly, the objective of this work was to elucidate the role of monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion protein 1 (VCAM-1) and asymmetric dimethylarginine (ADMA), key molecules in endothelial dysfunction, in the pathological processes of both underlying vascular damage and pulmonary fibrosis in RA-ILD.
Methods: Peripheral venous blood was collected from 21 patients with RA-ILD+ and individuals from 3 comparative groups: 25 patients with RA-ILD-, 21 patients with idiopathic pulmonary fibrosis (IPF) and 21 healthy controls (HC). All the individuals were recruited from the Rheumatology and Pneumology departments of Hospital Universitario Marqués de Valdecilla, Santander, Spain. Serum levels of MCP-1, VCAM-1 and ADMA were measured by enzyme-linked immunosorbent assay.
Results: Patients with RA-ILD+ exhibited significantly higher serum levels of MCP-1, VCAM-1 and ADMA in relation to HC (p< 0.001 in all cases, Figure 1A-C). Likewise, increased levels of MCP-1, VCAM-1 and ADMA were found in patients with RA-ILD+ compared to those with RA-ILD- (p< 0.001, p< 0.001 and p=0.023 respectively, Figure 1A-C). It was also the case when patients with RA-ILD+ were compared with patients with IPF (p< 0.001, p< 0.001 and p=0.001, respectively, Figure 1A-C).
Conclusion: Our study supports a role of MCP-1, VCAM-1 and ADMA in the underlying vasculopathy in RA-ILD+. Interestingly, an increase of serum levels of MCP-1, VCAM-1 and ADMA may help to identify the presence of ILD in patients with RA and to discriminate RA-ILD+ patients from those with IPF.
References: [1] J Clin Med 2019;8(12):2038; [2] Stem Cell Rev Rep 2018;14(2):223-235; [3] Atherosclerosis 2012;224(2):309-317.Personal funds, VP-C and SR-M: RD16/0012/0009(ISCIII-ERDF); FG: RICORS Program (RD21/0002/0025) (ISCIII-EU); RL-M: CP16/00033 (ISCIII-ESF).
 Figure 1. Serum levels of MCP-1 (A), VCAM-1 (B) and ADMA (C) in all the individuals of the study. Significant results are highlighted in bold.
Figure 1. Serum levels of MCP-1 (A), VCAM-1 (B) and ADMA (C) in all the individuals of the study. Significant results are highlighted in bold.Disclosures: V. Pulito-Cueto, None; S. Remuzgo-Martinez, None; F. Genre, None; B. Atienza-Mateo, AbbVie/Abbott, Roche, Pfizer, Celgene, Novartis, Janssen, UCB, Eli Lilly; V. Mora-Cuesta, None; D. Iturbe-Fernández, None; L. Lera-Gómez, None; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; V. Portilla, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi; A. Corrales, None; O. Gualillo, None; J. Cifrián, None; R. Lopez Mejias, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD.

