Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0372–0402) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster I
0380: Changes in Disease Activity and Patient-Reported Outcomes in Psoriatic Arthritis Patients Treated with Ixekizumab in a Real-World US Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

William Tillett, MD, PhD
Royal National Hospital for rheumatic diseases
Bath, United Kingdom
Abstract Poster Presenter(s)
William Tillett1, Julie Birt2, Cristi Cavanaugh3, Yoojin Jung3, Aisha Vadhariya2, Sarah Ross2, Jessica Paulus3, Aubrey Sprabery2 and Ennio Lubrano4, 1Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom, 2Eli Lilly and Company, Indianapolis, IN, 3OM1, Inc., Boston, MA, 4Academic Rheumatology Unit, Dipartimento di Medicina e Scienze della Salute ‘‘Vincenzo Tiberio’’, Università degli Studi del Molise, Campobasso, Italy
Background/Purpose: Ixekizumab (IXE), an IL-17A inhibitor, has demonstrated efficacy in clinical trials1-3 but real-world effectiveness data are limited.4 This study describes changes in disease activity and patient-reported outcomes (PROs) at 6 and 12 months follow-up among psoriatic arthritis (PsA) patients initiating IXE in a real-world setting.
Methods: This retrospective cohort study included patients from the OM1 PsA Registry (OM1, Boston, MA), a linked electronic medical record and administrative claims dataset with over 50,000 patients. Eligible patients had ≥1 prescription for IXE (first considered index), were ≥18 years old at index, had ≥1 diagnosis code for PsA in the 12 months before or on index, and had ≥12 months of baseline and ≥6 months of follow-up data as of June 2021. For patients with baseline and follow-up measures available, changes in Clinical Disease Activity Index (CDAI), PROs, and other clinical outcomes from baseline to 6 and 12 months were described. For patients on IXE monotherapy, change in CDAI score from baseline to 6 and 12 months was assessed using mixed effects linear models adjusted for age, sex, and baseline CDAI score.
Results: The study population included 1,812 patients with a mean age of 53.7 years (Table 1). For all patients, domains of PsA included psoriasis (82%) and enthesitis (28%). Over 60% of patients were obese, and the mean Charlson Comorbidity Index was 1.3. Of 291 patients with a baseline CDAI score, 61% had moderate or severe disease activity. Most patients (84%) had prior treatment with a biologic disease-modifying antirheumatic drug (bDMARD) and 40% with a targeted synthetic DMARD (tsDMARD). The mean number of prior bDMARDs and tsDMARDs used during all available prior history was 2.3 and 1.1, respectively. The most common prior b/tsDMARDs were secukinumab (n=428, 24%) and adalimumab (n=245, 14%). For all patients, CDAI scores improved (decreased) by an average of 3.4 and 3.7 points at 6 and 12 months, respectively, from a baseline mean of 15.4. All disease activity measures and PROs improved from baseline to 6 and 12 months (Figure 1). In patients persistent with IXE, 35.3% and 33.7% were in CDAI remission or low disease activity at 6 and 12 months after initiation, respectively. For IXE monotherapy users (82% of patients), at baseline, patients had a mean CDAI of 14.3 (n=131) and 15.1 (n=105) for the 6 and12 month analyses, respectively. Adjusted mean changes in CDAI from baseline to 6 months (-3.6 points, p < 0.0001) and 12 months (-4.9 points, p < 0.0001) were statistically significant.
Conclusion: In this cohort of biologic-experienced, difficult to treat PsA patients with high comorbidity burden, improvements in disease activity and PROs were observed at 6 and 12 months after initiating treatment with IXE. Improvements were observed in patients overall and in the monotherapy subgroup. More real-world research on IXE and other bDMARDs may inform the effect of treatment choices on clinical and PROs in both bDMARD-naive and experienced PsA patients.
References:
[1] Mease PJ. Ann. Rheum. Dis. 2017;76(1):79-87.
[2] Nash P. Lancet. 2017;389(10086):2317-2327.
[3] Mease PJ. Ann. Rheum. Dis. 2020;79(1):123-131.
[4] Berman J. Biologics. 2021 Nov 18;15:463-470.<
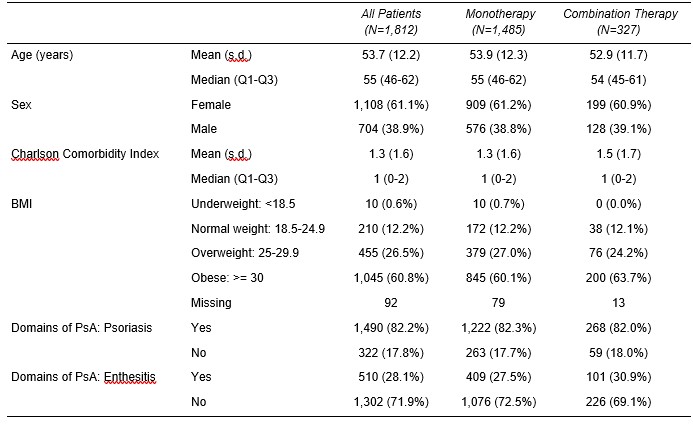 Table 1. Demographic and Clinical Characteristics by Therapy Status
Table 1. Demographic and Clinical Characteristics by Therapy Status
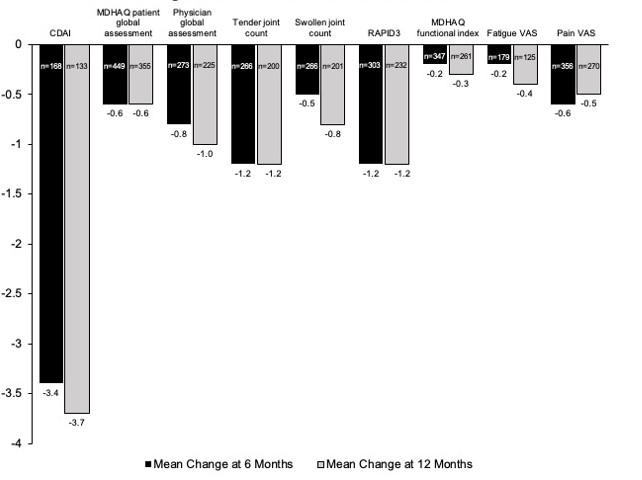 Figure 1: Clinical Outcomes at 6 and 12 Months
Figure 1: Clinical Outcomes at 6 and 12 Months
Disclosures: W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; J. Birt, Eli Lilly and Company; C. Cavanaugh, None; Y. Jung, None; A. Vadhariya, Eli Lilly and Company; S. Ross, Eli Lilly and Company; J. Paulus, None; A. Sprabery, Eli Lilly and Company; E. Lubrano, None.
Background/Purpose: Ixekizumab (IXE), an IL-17A inhibitor, has demonstrated efficacy in clinical trials1-3 but real-world effectiveness data are limited.4 This study describes changes in disease activity and patient-reported outcomes (PROs) at 6 and 12 months follow-up among psoriatic arthritis (PsA) patients initiating IXE in a real-world setting.
Methods: This retrospective cohort study included patients from the OM1 PsA Registry (OM1, Boston, MA), a linked electronic medical record and administrative claims dataset with over 50,000 patients. Eligible patients had ≥1 prescription for IXE (first considered index), were ≥18 years old at index, had ≥1 diagnosis code for PsA in the 12 months before or on index, and had ≥12 months of baseline and ≥6 months of follow-up data as of June 2021. For patients with baseline and follow-up measures available, changes in Clinical Disease Activity Index (CDAI), PROs, and other clinical outcomes from baseline to 6 and 12 months were described. For patients on IXE monotherapy, change in CDAI score from baseline to 6 and 12 months was assessed using mixed effects linear models adjusted for age, sex, and baseline CDAI score.
Results: The study population included 1,812 patients with a mean age of 53.7 years (Table 1). For all patients, domains of PsA included psoriasis (82%) and enthesitis (28%). Over 60% of patients were obese, and the mean Charlson Comorbidity Index was 1.3. Of 291 patients with a baseline CDAI score, 61% had moderate or severe disease activity. Most patients (84%) had prior treatment with a biologic disease-modifying antirheumatic drug (bDMARD) and 40% with a targeted synthetic DMARD (tsDMARD). The mean number of prior bDMARDs and tsDMARDs used during all available prior history was 2.3 and 1.1, respectively. The most common prior b/tsDMARDs were secukinumab (n=428, 24%) and adalimumab (n=245, 14%). For all patients, CDAI scores improved (decreased) by an average of 3.4 and 3.7 points at 6 and 12 months, respectively, from a baseline mean of 15.4. All disease activity measures and PROs improved from baseline to 6 and 12 months (Figure 1). In patients persistent with IXE, 35.3% and 33.7% were in CDAI remission or low disease activity at 6 and 12 months after initiation, respectively. For IXE monotherapy users (82% of patients), at baseline, patients had a mean CDAI of 14.3 (n=131) and 15.1 (n=105) for the 6 and12 month analyses, respectively. Adjusted mean changes in CDAI from baseline to 6 months (-3.6 points, p < 0.0001) and 12 months (-4.9 points, p < 0.0001) were statistically significant.
Conclusion: In this cohort of biologic-experienced, difficult to treat PsA patients with high comorbidity burden, improvements in disease activity and PROs were observed at 6 and 12 months after initiating treatment with IXE. Improvements were observed in patients overall and in the monotherapy subgroup. More real-world research on IXE and other bDMARDs may inform the effect of treatment choices on clinical and PROs in both bDMARD-naive and experienced PsA patients.
References:
[1] Mease PJ. Ann. Rheum. Dis. 2017;76(1):79-87.
[2] Nash P. Lancet. 2017;389(10086):2317-2327.
[3] Mease PJ. Ann. Rheum. Dis. 2020;79(1):123-131.
[4] Berman J. Biologics. 2021 Nov 18;15:463-470.<
 Table 1. Demographic and Clinical Characteristics by Therapy Status
Table 1. Demographic and Clinical Characteristics by Therapy Status  Figure 1: Clinical Outcomes at 6 and 12 Months
Figure 1: Clinical Outcomes at 6 and 12 MonthsDisclosures: W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; J. Birt, Eli Lilly and Company; C. Cavanaugh, None; Y. Jung, None; A. Vadhariya, Eli Lilly and Company; S. Ross, Eli Lilly and Company; J. Paulus, None; A. Sprabery, Eli Lilly and Company; E. Lubrano, None.

