Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - IBD
45 - Persistence of Antibodies Six Months After Three COVID-19 mRNA Vaccine Doses in Patients with Inflammatory Bowel Disease (Late-Breaking Abstract)
Tuesday, October 25, 2022
3:35 PM – 3:45 PM ET
Location: Hall C2

Mazen Almasry, MBBS
University of Wisconsin School of Medicine and Public Health
Madison, WI
Late Breaking Abstract Presenter(s)
Mazen Almasry, MBBS1, Luke Richard, MD1, Francis A. Farraye, MD, MSc, MACG2, Mary S. Hayney, PharmD, MPH, 3 Freddy Caldera, DO, MS, FACG1; 1University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI; 2Mayo Clinic, Jacksonville, FL; 3University of Wisconsin-Madison School of Pharmacy, Madison, WI
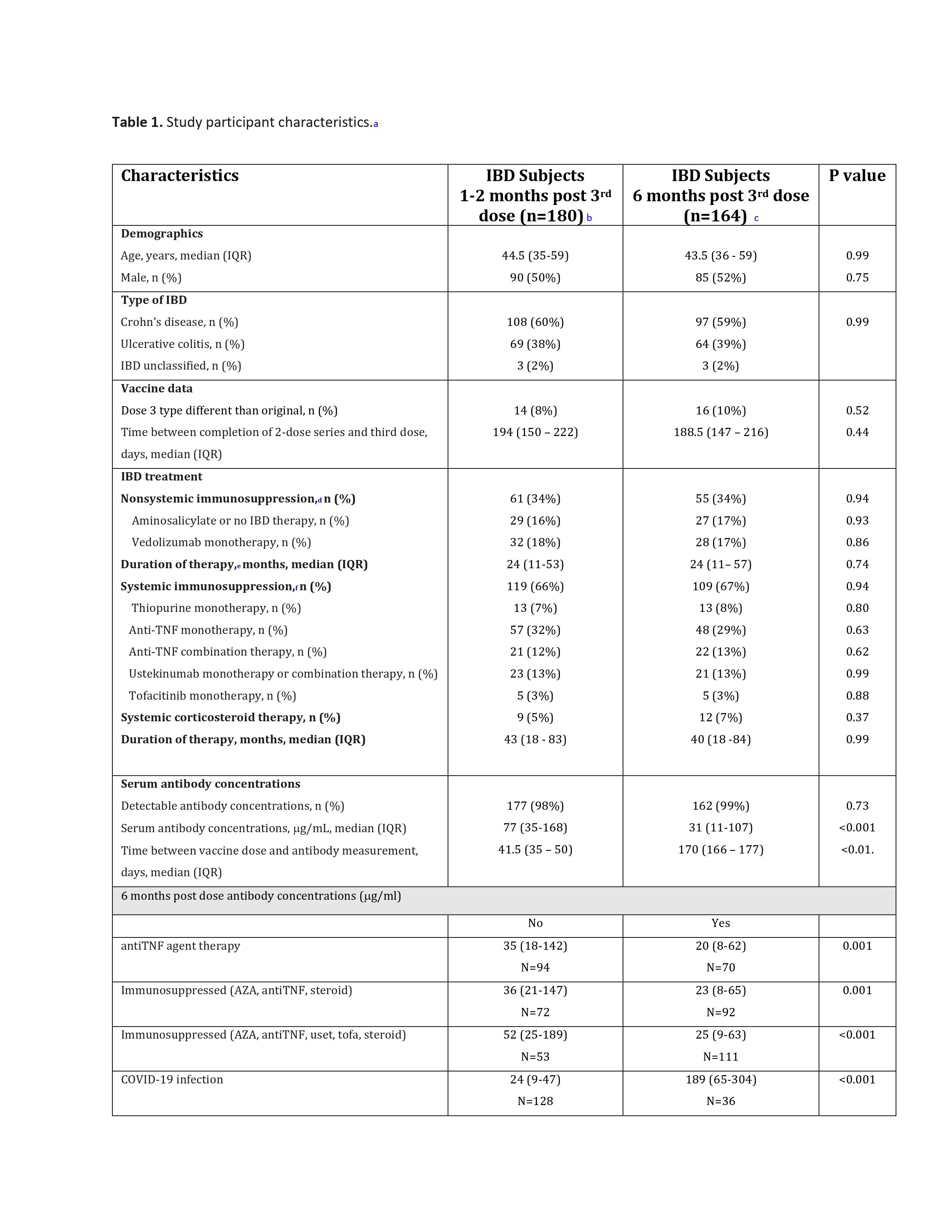
Introduction: Studies evaluating immunogenicity of COVID-19 vaccines in patients with Inflammatory Bowel Disease (IBD) have demonstrated greater than 99% response rate after three doses of the mRNA COVID-19 vaccine series. Yet, contemporary data on the persistence of antibody levels after three doses is lacking. The aim of this study was to evaluate the sustained antibody concentrations in IBD patients six months after a third COVID-19 mRNA vaccine dose.
Methods: Humoral and Cellular Initial and Sustained Immunogenicity in Patients with IBD (HERCULES) is a prospective study evaluating the humoral immunogenicity of COVID-19 vaccines. Eligible participants included patients with a diagnosis of IBD, ages 18-85 years, on stable doses of maintenance therapy for at least 2 months, and had received three doses of mRNA vaccines confirmed using the Wisconsin Immunization Registry.
Serum SARS-CoV-2 anti-spike IgG antibody concentrations were measured at one month (t1) and six months (t2) after a third dose of an mRNA COVID-19 vaccine. A small cohort had concentrations available one month (t3) after a fourth dose. The primary outcome was antibody concentrations at (t2) compared to (t1). Secondary outcomes included evaluating the effects of immune modifying therapies on sustained antibody concentrations, and previous COVID-19 infection at (t2). Secondary outcomes also included antibody concentrations at (t3) compared to (t2).
Results: 180 patients had antibody concentration measured at (t1), 164 at (t2) and 49 at (t3). 98.7% of individuals (162/164) had measurable antibodies at (t2), and 100% (49/49) had measurable antibodies at (t3). Antibody concentrations were significantly lower at (t2) 31 mcg/ml compared to at (t1) 77 mcg/ml (p<0.001). Those on anti-TNF therapy had lower antibody concentrations compared to those on non-anti-TNF regimens (median 20 mcg/ml vs. 35 mcg/ml; p=0.001). However, prior COVID-19 infection resulted in higher antibody concentrations (median 189 mcg/ml vs. 24 mcg/ml; p<0.001). Antibody concentrations were higher at (t3) 98 mcg/ml compared to at (t2) 31 mcg/ml (p<0.001).
Discussion: Almost all patients with IBD had measurable antibodies six months after a third dose of an mRNA COVID-19 vaccine. Antibodies were also present in all of those who had received a fourth dose. Those on anti-TNF therapy had lower antibody concentrations which is consistent with prior studies. Studies continue to be needed to monitor responses to additional doses and the expected bivalent vaccines.
Disclosures:
All authors have indicated no relevant financial relationships.
Mazen Almasry, MBBS, Luke Richard, MD, Francis A. Farraye, MD, MSc, MACG, Mary S. Hayney, PharmD, MPH, Freddy Caldera, DO, MS, FACG, 45, Persistence of Antibodies Six Months After Three COVID-19 mRNA Vaccine Doses in Patients with Inflammatory Bowel Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Introduction: Studies evaluating immunogenicity of COVID-19 vaccines in patients with Inflammatory Bowel Disease (IBD) have demonstrated greater than 99% response rate after three doses of the mRNA COVID-19 vaccine series. Yet, contemporary data on the persistence of antibody levels after three doses is lacking. The aim of this study was to evaluate the sustained antibody concentrations in IBD patients six months after a third COVID-19 mRNA vaccine dose.
Methods: Humoral and Cellular Initial and Sustained Immunogenicity in Patients with IBD (HERCULES) is a prospective study evaluating the humoral immunogenicity of COVID-19 vaccines. Eligible participants included patients with a diagnosis of IBD, ages 18-85 years, on stable doses of maintenance therapy for at least 2 months, and had received three doses of mRNA vaccines confirmed using the Wisconsin Immunization Registry.
Serum SARS-CoV-2 anti-spike IgG antibody concentrations were measured at one month (t1) and six months (t2) after a third dose of an mRNA COVID-19 vaccine. A small cohort had concentrations available one month (t3) after a fourth dose. The primary outcome was antibody concentrations at (t2) compared to (t1). Secondary outcomes included evaluating the effects of immune modifying therapies on sustained antibody concentrations, and previous COVID-19 infection at (t2). Secondary outcomes also included antibody concentrations at (t3) compared to (t2).
Results: 180 patients had antibody concentration measured at (t1), 164 at (t2) and 49 at (t3). 98.7% of individuals (162/164) had measurable antibodies at (t2), and 100% (49/49) had measurable antibodies at (t3). Antibody concentrations were significantly lower at (t2) 31 mcg/ml compared to at (t1) 77 mcg/ml (p<0.001). Those on anti-TNF therapy had lower antibody concentrations compared to those on non-anti-TNF regimens (median 20 mcg/ml vs. 35 mcg/ml; p=0.001). However, prior COVID-19 infection resulted in higher antibody concentrations (median 189 mcg/ml vs. 24 mcg/ml; p<0.001). Antibody concentrations were higher at (t3) 98 mcg/ml compared to at (t2) 31 mcg/ml (p<0.001).
Discussion: Almost all patients with IBD had measurable antibodies six months after a third dose of an mRNA COVID-19 vaccine. Antibodies were also present in all of those who had received a fourth dose. Those on anti-TNF therapy had lower antibody concentrations which is consistent with prior studies. Studies continue to be needed to monitor responses to additional doses and the expected bivalent vaccines.

Disclosures:
All authors have indicated no relevant financial relationships.
Mazen Almasry, MBBS, Luke Richard, MD, Francis A. Farraye, MD, MSc, MACG, Mary S. Hayney, PharmD, MPH, Freddy Caldera, DO, MS, FACG, 45, Persistence of Antibodies Six Months After Three COVID-19 mRNA Vaccine Doses in Patients with Inflammatory Bowel Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

