Oral Paper Presentation
Annual Scientific Meeting
63 - An Open-Label Study (ECOSPOR IV) to Evaluate the Safety, Efficacy and Durability of SER-109, an Investigational Oral Microbiome Therapeutic, in Adults With Recurrent Clostridioides difficile Infection (rCDI)

Sahil Khanna, MBBS, MS, FACG
Mayo Clinic
Rochester, MN
Presenting Author(s)
1Mayo Clinic, Rochester, MN; 2PACT Gastroenterology Center and Yale University School of Medicine, New Haven, CT; 3Sutter Health Research Enterprise, Mountain View, CA; 4Vanguard Gastroenterology, New York, NY; 5Seres Therapeutics, Cambridge, MA
Introduction: Antibiotics are often insufficient to treat patients with rCDI due to persistence of spores and microbiome disruption. Recurrence occurs in 20-36% of patients with 1st recurrence and ≥40% in those with ≥2 recurrences. We reported that SER-109, an investigational, oral microbiome therapeutic comprised of purified Firmicutes spores, was superior to placebo in reducing risk of rCDI at 8 weeks (12% vs 40%, respectively) in patients with ≥2 CDI recurrences (Feuerstadt, P. N Engl J Med 2022). Here, we report the results of ECOSPOR IV, an open-label trial of SER-109.
Methods: Subjects with rCDI were enrolled at 72 US/Canadian sites in 2 cohorts: a) rollover subjects with rCDI in ECOSPOR III, diagnosed by toxin EIA) and b) subjects with ≥1 CDI recurrence (diagnosed by PCR or toxin EIA), inclusive of the current episode). After standard-of-care antibiotics, subjects received SER-109 (4 capsules daily x 3 days). Efficacy, including the proportion of subjects with rCDI (toxin+ diarrhea requiring treatment), and safety were evaluated through Week 8; durability of response was assessed through Week 24.
Results: Of 351 subjects screened, 263 were enrolled (Cohort 1: N=29; Cohort 2: N=234; 68% female; mean age 64 years). Comorbidities included cardiac disorders (31%), neoplasms (21%), Type 2 diabetes (11%), COPD (10%), chronic kidney disease (9%), and hepatobiliary disorders (9%). Overall, 137 subjects (52.1%) experienced treatment-emergent adverse events (TEAEs) through Week 8. The majority were mild to moderate in intensity and gastrointestinal (Table 1). There were 6 deaths (2.3%) and 20 subjects (7.6%) with serious TEAEs, none of which were deemed treatment-related.
Overall, rCDI occurred in 23 subjects (8.7%) at Week 8 (4/29 in Cohort 1 [13.8%] and 19/234 in Cohort 2 [8.1%]) and rCDI rates remained low through 24 weeks (13.7% [36/263]). CDI recurrence rates at Week 8 in subjects with first recurrence were similarly low (6.5% [5/77]) as those with ≥2 recurrences (9.7% [18/186]; Figure 1).
Discussion: SER-109, a potential first-in-class investigational microbiome therapeutic, was observed to be safe and well-tolerated in this population of older patients with multiple comorbidities. The rate of rCDI was low and durable, regardless of the number of prior episodes, supporting the potential benefit of microbiome repair following antibiotics for rCDI. Earlier intervention with SER-109 in first recurrence may reduce morbidity associated with rCDI.
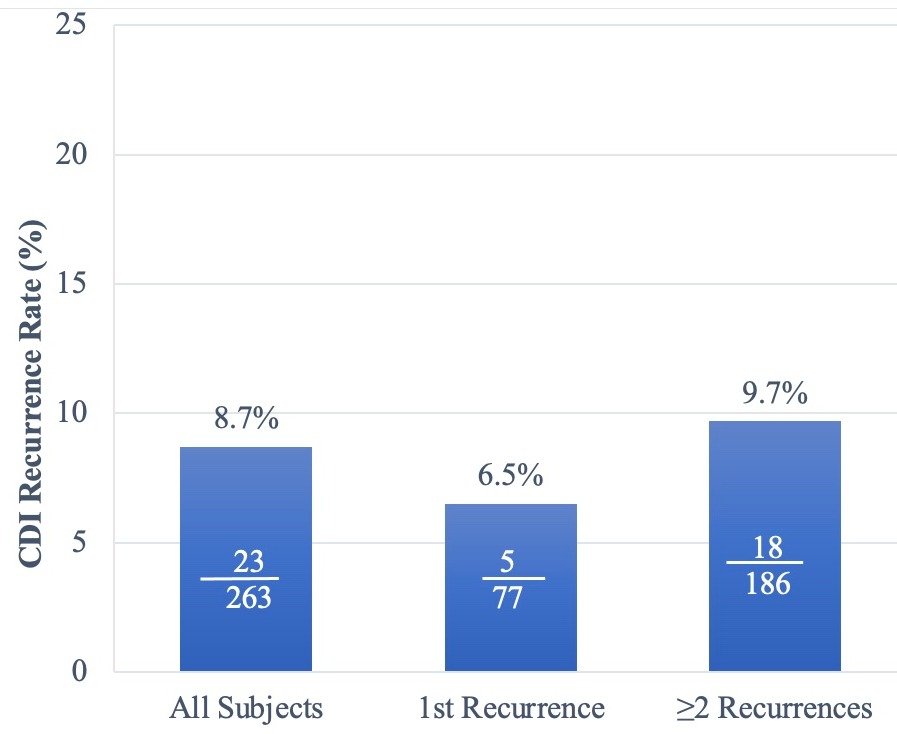
Note: Subjects who are lost to follow-up, terminated the study prematurely, or died without a recorded recurrence before the end of the time interval are assumed to have had a recurrence.
Numbers presented within each bar represent the number of subjects with CDI recurrence over the total number of subjects within each grouping.
| Cohort 1 | Cohort 2 (N=234) n (%) | Total | ||
| Randomized Treatment Arm in ECOSPOR III | Total | |||
| SER-109 | Placebo | |||
Any TEAE | 4 (100.0) | 15 (60.0) | 19 (65.5) | 118 (50.4) | 137 (52.1) |
Most Frequently Reported TEAEs by Preferred Term (≥5% in any cohort) |
|
|
|
|
|
Diarrhoea | 1 (25.0) | 9 (36.0) | 10 (34.5) | 49 (20.9) | 59 (22.4) |
Flatulence | 0 | 4 (16.0) | 4 (13.8) | 16 (6.8) | 20 (7.6) |
Nausea | 0 | 3 (12.0) | 3 (10.3) | 17 (7.3) | 20 (7.6) |
Abdominal pain | 1 (25.0) | 2 (8.0) | 3 (10.3) | 15 (6.4) | 18 (6.8) |
Fatigue | 0 | 3 (12.0) | 3 (10.3) | 9 (3.8) | 12 (4.6) |
Urinary tract infection | 0 | 0 | 0 | 12 (5.1) | 12 (4.6) |
Abdominal distension | 1 (25.0) | 3 (12.0) | 4 (13.8) | 7 (3.0) | 11 (4.2) |
Related/Possibly Related TEAE | 1 (25.0) | 4 (16.0) | 5 (17.2) | 27 (11.5) | 32 (12.2) |
Severe TEAEs | 0 | 1 (4.0) | 1 (3.4) | 18 (7.7) | 19 (7.2) |
Serious TEAEs | 0 | 0 | 0 | 20 (8.5) | 20 (7.6) |
Serious TEAEs Related/Possibly Related to Study Drug | 0 | 0 | 0 | 0 | 0 |
Treatment-emergent AESIs | 0 | 0 | 0 | 10 (4.3) | 10 (3.8) |
TEAEs Leading to Death1 | 0 | 0 | 0 | 6 (2.6) | 6 (2.3) |
Abbreviations: AESI = adverse event of special interest; TEAE = treatment-emergent adverse event
Notes: Data presented are by subject. All TEAEs were collected and summarized from time of enrollment up to Week 8; Note: N is number of subjects in the Safety Population who are in the study at the beginning of the specified time interval.
1 TEAEs leading to death by preferred term included: congestive cardiomyopathy (1 subject), coronavirus infection and intestinal peforation (1 subect), death due to natural causes (1 subject), clostridium difficile infection (1 subject), necrotising fasciitis (1 subject), and pancreatic carcinoma (1 subject). Note: the start date of the fatal SAE of pancreatic carcinoma began prior to Week 8 with fatal outcome occurred after Week 12. No deaths were deemed by the investigator to be related to study drug.
Disclosures:
Sahil Khanna, MBBS, MS, FACG1, Paul Feuerstadt, MD, FACG2, Edward Huang, MD, MPH3, Caterina Oneto, MD4, Darrell S. Pardi, MD, MS, FACG1, Elaine E. Wang, MD5, Ananya De, PhD5, Kelly Brady, MD5, Asli Memisoglu, PhD5, David Lombardi, PhD5, Brooke Hasson, PhD5, Barbara McGovern, MD5, Lisa Von Moltke, MD5, 63, An Open-Label Study (ECOSPOR IV) to Evaluate the Safety, Efficacy and Durability of SER-109, an Investigational Oral Microbiome Therapeutic, in Adults With Recurrent Clostridioides difficile Infection (rCDI), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.


