Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - IBD
44 - Efficacy and Safety of Upadacitinib Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Results from a Randomized Phase 3 U-ENDURE Maintenance Study (Late-Breaking Abstract)
Tuesday, October 25, 2022
3:25 PM – 3:35 PM ET
Location: Hall C2

Edward V. Loftus, Jr., MD, FACG
Professor of Medicine
Division of Gastroenterology and Hepatology, Mayo Clinic
Rochester, MN
Late Breaking Abstract Presenter(s)
Julian Panes,1 Edward V. Loftus Jr., MD, FACG,2 Ana Lacerda,3 Laurent Peyrin-Biroulet,4 Geert D'Haens,5 Remo Panaccione,6 Walter Reinisch,7 Edouard Louis,8 Minhu Chen,9 Hiroshi Nakase,10 Jakob Begun,11 Brigid S. Boland,12 Jianzhong Liu,3 Elena Dubcenco,3 Mohamed-Eslam F. Mohamed,3 Tian Feng,3 Jean-Frederic Colombel,13; 1Hospital Clinic Barcelona, IDIPABS, CIBERehd, Barcelona; 2Mayo Clinic College of Medicine and Science, Rochester, MN; 3AbbVie Inc, North Chicago, IL; 4University Hospital of Nancy, Lorraine University, Vandoeuvre, France; 5Amsterdam University Medical Centres, Amsterdam, Netherlands; 6University of Calgary, Calgary, Alberta, Canada; 7Medical University of Vienna, Vienna, Austria; 8University Hospital CHU of Liège, Liège, Belgium; 9The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; 10Sapporo Medical University School of Medicine, Sapporo, Japan; 11Mater Hospital Brisbane, Brisbane, QLD Australia; 12University of California, San Diego, San Diego, CA; 13Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Efficacy and safety of upadacitinib (UPA) 15mg(UPA15) once daily(QD) and UPA 30mg QD(UPA30) maintenance in patients (pts) with Crohn’s disease (CD) were evaluated in U-ENDURE.
Methods: Pts with moderate to severe CD who responded(>30% decrease average daily very soft/liquid SF and/or average daily APS, neither greater than baseline) to UPA 45mg QD over 12weeks(wks) in one induction studies were eligible for U-ENDURE. At wk12, pts were randomized(1:1:1) to UPA30, UPA15 or PBO for 52wks maintenance therapy.1 Co-primary endpoints, clinical remission per CD activity index(CDAI, US) or SF/APS(EU) and endoscopic response were evaluated at wk52. Safety, clinical, and endoscopic outcomes were evaluated at or through wk52.
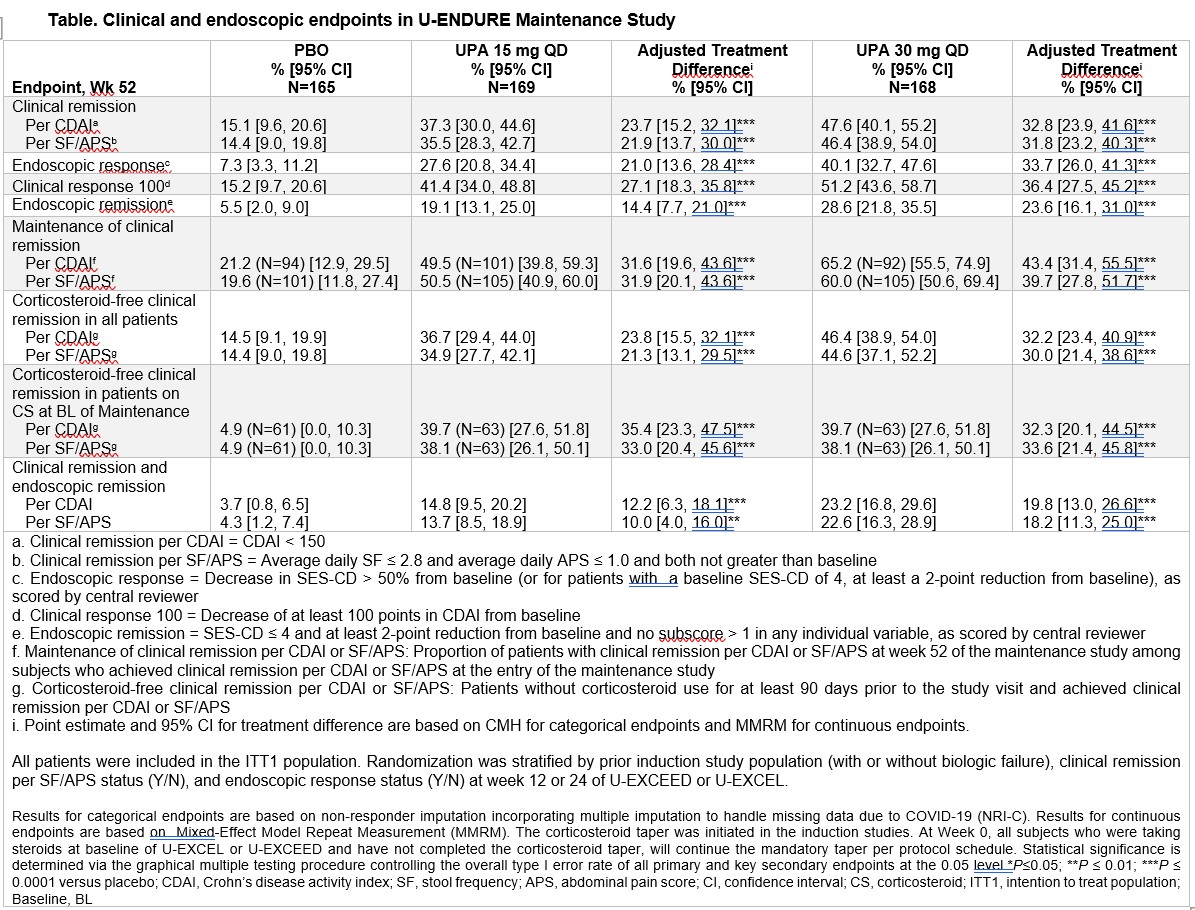
Results: Baseline characteristics were similar between groups; 75% of pts failed prior biologic. At wk52, greater proportion of pts receiving UPA15(n=169) & UPA30(n=168) achieved clinical remission per CDAI(UPA15 37.3% & UPA30 47.6% vs PBO 15.1%) and per SF/APS(UPA15 35.5% & UPA30 46.4% vs PBO 14.4%), P<0.0001 for all comparisons. UPA15 and UPA30 pts attained greater rates of endoscopic response (UPA15 27.6% & UPA30 40.1% vs PBO 7.3%, P<0.0001 for both comparisons). UPA15 and UPA30 were superior to PBO for key secondary endpoints including clinical response, endoscopic remission, maintenance of clinical remission, corticosteroid-free clinical remission, and clinical and endoscopic remission. Rates of adverse events (AE) and serious AEs were similar across UPA groups; AE leading to treatment discontinuation rates were similar across groups. Most common AE was CD worsening(58.0 events/100 pt-years[E/100PY] in PBO, (29.7E/100PY)UPA15, and (12.0E/100PY)UPA30. Serious infection rates were similar across groups (6.1-8.4 E/100PY); herpes zoster rate was higher in UPA30(7.2E/100PY) vs PBO(4.7E/100PY) and UPA15(4.0E/100PY). Malignancies excluding non-melanoma skin cancer (NMSC) were reported in 1 pt UPA15 pt and2 UPA30 pts; all events were diagnosed within 9months of first UPA exposure. One gastrointestinal perforation was reported in each group. One event of hepatic vein thrombosis was reported in UPA30. No deaths, tuberculosis, NMSC, or adjudicated cardiovascular events occurred in any group.
Conclusion: Among pts with moderate to severe active CD who respond to UPA induction therapy, maintenance treatment with UPA15 and UPA30 was superior to PBO for all clinical and endoscopic outcomes at wk52. UPA15 and UPA30 were well tolerated, safety profiles comparable to previous UPA studies.
Disclosures:
Julian Panes, Edward V. Loftus Jr., MD, FACG, Ana Lacerda, Laurent Peyrin-Biroulet, Geert D'Haens, Remo Panaccione, Walter Reinisch, Edouard Louis, Minhu Chen, Hiroshi Nakase, Jakob Begun, Brigid S. Boland, Jianzhong Liu, Elena Dubcenco, Mohamed-Eslam F. Mohamed, Tian Feng, Jean-Frederic Colombel, 44, Efficacy and Safety of Upadacitinib Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Results from a Randomized Phase 3 U-ENDURE Maintenance Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Introduction: Efficacy and safety of upadacitinib (UPA) 15mg(UPA15) once daily(QD) and UPA 30mg QD(UPA30) maintenance in patients (pts) with Crohn’s disease (CD) were evaluated in U-ENDURE.
Methods: Pts with moderate to severe CD who responded(>30% decrease average daily very soft/liquid SF and/or average daily APS, neither greater than baseline) to UPA 45mg QD over 12weeks(wks) in one induction studies were eligible for U-ENDURE. At wk12, pts were randomized(1:1:1) to UPA30, UPA15 or PBO for 52wks maintenance therapy.1 Co-primary endpoints, clinical remission per CD activity index(CDAI, US) or SF/APS(EU) and endoscopic response were evaluated at wk52. Safety, clinical, and endoscopic outcomes were evaluated at or through wk52.
Results: Baseline characteristics were similar between groups; 75% of pts failed prior biologic. At wk52, greater proportion of pts receiving UPA15(n=169) & UPA30(n=168) achieved clinical remission per CDAI(UPA15 37.3% & UPA30 47.6% vs PBO 15.1%) and per SF/APS(UPA15 35.5% & UPA30 46.4% vs PBO 14.4%), P<0.0001 for all comparisons. UPA15 and UPA30 pts attained greater rates of endoscopic response (UPA15 27.6% & UPA30 40.1% vs PBO 7.3%, P<0.0001 for both comparisons). UPA15 and UPA30 were superior to PBO for key secondary endpoints including clinical response, endoscopic remission, maintenance of clinical remission, corticosteroid-free clinical remission, and clinical and endoscopic remission. Rates of adverse events (AE) and serious AEs were similar across UPA groups; AE leading to treatment discontinuation rates were similar across groups. Most common AE was CD worsening(58.0 events/100 pt-years[E/100PY] in PBO, (29.7E/100PY)UPA15, and (12.0E/100PY)UPA30. Serious infection rates were similar across groups (6.1-8.4 E/100PY); herpes zoster rate was higher in UPA30(7.2E/100PY) vs PBO(4.7E/100PY) and UPA15(4.0E/100PY). Malignancies excluding non-melanoma skin cancer (NMSC) were reported in 1 pt UPA15 pt and2 UPA30 pts; all events were diagnosed within 9months of first UPA exposure. One gastrointestinal perforation was reported in each group. One event of hepatic vein thrombosis was reported in UPA30. No deaths, tuberculosis, NMSC, or adjudicated cardiovascular events occurred in any group.
Conclusion: Among pts with moderate to severe active CD who respond to UPA induction therapy, maintenance treatment with UPA15 and UPA30 was superior to PBO for all clinical and endoscopic outcomes at wk52. UPA15 and UPA30 were well tolerated, safety profiles comparable to previous UPA studies.

Disclosures:
Julian Panes received financial support for research from AbbVie and Pfizer; received lecture fee(s) from AbbVie, Ferring, Janssen, Takeda; and received consultancy fees from AbbVie, Arena Pharmaceuticals, Athos, Boehringer Ingelheim, Celltrion, Ferring, Galapagos, Genentech, Glaxo Smith Kline, Janssen, Mirum, Morphic, Origo, Pandion, Pfizer, Protagonist, Revolo, Robarts, Roche, Takeda, Theravance, and Wassermann.
Edward V. Loftus Jr. has received consulting fees from AbbVie, UCB, Janssen, Takeda, Celgene, Eli Lilly, Amgen, Protagonist, Pfizer, Scipher Medicine, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Gilead, Gossamer Bio, Arena, Calibr, Iterative Scopes, Morphic Therapeutics, Ono Pharma, Surrozen, Sun Pharma, and Fresenius Kabi; research support from AbbVie, UCB, Genentech, Janssen, Pfizer, Takeda, Robarts Clinical Trials, Gilead, Celgene, Receptos, Bristol-Myers Squibb, Theravance, and Gossamer Bio.
Laurent Peyrin-Biroulet has received personal fees from AbbVie, Allergan, Alma Bio Therapeutics, Amgen, Arena, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring, Genentech, Gilead, Hikma, Index Pharmaceuticals, Janssen, Merck, Nestlé, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna Biological, Takeda, and Tillotts Pharma; and grants from AbbVie, Merck, and Takeda. He also holds Clinical Trials Mobile Application (CTMA) stock options.
Geert D'Haens has served as an adviser for AbbVie, Ablynx, Allergan, Alphabiomics, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meyers Squibb, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Echo Pharmaceuticals, Eli Lilly, Engene, Ferring, DrFALK Pharma, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Hospira/Pfizer, Immunic, Johnson and Johnson, Kintai Therapeutics, Lycera, Medimetrics, Millenium/Takeda, Medtronics, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prodigest, Prometheus laboratories/Nestle, Progenity, Protagonist, RedHill, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor, and has received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor.
Remo Panaccione has received consulting fees, speaker fees, and research support from AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB, and Takeda Pharmaceuticalsz.
Walter Reinisch has served as a speaker for Abbvie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Medice, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, Yakult; as a consultant for Abbvie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Calyx, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Landos Biopharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Teva Pharma, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; as an advisory board member for Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC, and has received research funding from Abbvie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, Janssen, MSD, Sandoz, Takeda.
Edouard Louis has received research grants from Janssen, Pfizer, and Takeda; received educational grants from AbbVie, Janssen, MSD, and Takeda; received speaker fees from AbbVie, Falk, Ferring, Hospira, Janssen, MSD, Pfizer, and Takeda; served on advisory boards for AbbVie, Arena, Celgene, Ferring, Galapagos, Gilead, Hospira, Janssen, MSD, Pfizer, and Takeda; and served as a consultant for AbbVie.
Min-Hu Chen has received support for clinical research from Janssen and Takeda; and served as an advisory board member for Boehringer Ingelheim GmbH and Janssen; and provided educational activities for AbbVie, China Medical System, IPSEN, Janssen, and Takeda.
Hiroshi Nakase has received support from AbbVie GK., Kissei Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co.,Ltd., Mitsubishi Tanabe Pharma Corporation, Janssen Pharmaceutical K.K, Takeda Pharmaceutical Co.,Ltd., Pfizer Inc, Celgene Corporation., EA Pharma Co., Ltd., Zeria Pharmaceutical Co.,Ltd., Mochida Pharmaceutical Co.,Ltd., Nippon Kayaku Co.,Ltd., Daiichi Sankyo Company, Limited., JIMRO Co.,Ltd., as well as grants for commissioned/joint research from Hoya Group Pentax Medical, Boehringer Ingelheim GmbH, Bristol-Myers Squibb Company.
Jakob Begun has received consulting fees, speaker fees, and research support from AbbVie, Amgen, Anatara, AstraZeneca, Bristol-Myers Squibb, Celltrion, Eli Lilly, Ferring, Glaxo-Smith Kline, Janssen, Merck, Pfizer, Progenity, Roche, Sandoz, Suono, Takeda Pharmaceuticals.
Brigid S. Boland has received research grants from Prometheus Biosciences and Gilead; served on advisory boards for Takeda and Bristol-Myers Squibb.
Ana Lacerda, Jianzhong Liu, Elena Dubcenco, Mohamed-Eslam F. Mohamed and Tian Feng are employees of AbbVie and may own stock and/or options.
Jean-Frederic Colombel is a consultant and/or speaker for: AbbVie, Amgen, Allergan, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Shire, Takeda, TiGenix; receives research grants from: AbbVie, Janssen Pharmaceuticals and Takeda; stockholder: Intestinal Biotech Development.
Funding: AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Julian Panes, Edward V. Loftus Jr., MD, FACG, Ana Lacerda, Laurent Peyrin-Biroulet, Geert D'Haens, Remo Panaccione, Walter Reinisch, Edouard Louis, Minhu Chen, Hiroshi Nakase, Jakob Begun, Brigid S. Boland, Jianzhong Liu, Elena Dubcenco, Mohamed-Eslam F. Mohamed, Tian Feng, Jean-Frederic Colombel, 44, Efficacy and Safety of Upadacitinib Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Results from a Randomized Phase 3 U-ENDURE Maintenance Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

