Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 2
10 - An Objective Spatialomics Test Standardizes Management Decisions With Potential to Improve Health Outcomes for Barrett’s Esophagus Patients
Monday, October 24, 2022
10:18 AM – 10:30 AM ET
Location: Hall C2

Jacques Bergman, MD, PhD
Amsterdam University Medical Centres
Amsterdam, Noord-Holland, Netherlands
Presenting Author(s)
*Lucas C. Duits will deliver this presentation in Charlotte.*
Nicola F. F. Frei, MD1, Lucas C. Duits, MD, PhD2, Amir Khoshiwal, MD3, Roos E. Pouw, MD, PhD3, Christian Smolko, PhD4, Meenakshi Arora, PhD4, Jennifer J. Siegel, MA, PhD5, Rebecca Critchley-Thorne, PhD4, Jacques Bergman, MD, PhD6
1University Medical Center Amsterdam, Amsterdam, Noord-Holland, Netherlands; 2Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, Noord-Holland, Netherlands; 3Amsterdam UMC, Amsterdam, Noord-Holland, Netherlands; 4Castle Biosciences, Pittsburgh, PA; 5Castle Biosciences, Friendswood, TX; 6Amsterdam University Medical Centres, Amsterdam, Noord-Holland, Netherlands
Introduction: The diagnosis of low-grade dysplasia (LGD) in Barrett’s esophagus (BE) is associated with increased risk of progression to high grade dysplasia (HGD) or esophageal adenocarcinoma (EAC). However, due to substantial observer variability in the diagnosis of LGD, a patient’s management plan and health outcome depends largely on which pathologist reviews their case. Recent studies have shown that an objective spatialomics test (TissueCypher) predicts neoplastic progression in BE patients with higher sensitivity than pathology review. This study evaluated the test’s potential to augment pathology to standardize clinical management in a manner consistent with improved health outcomes for BE patients.
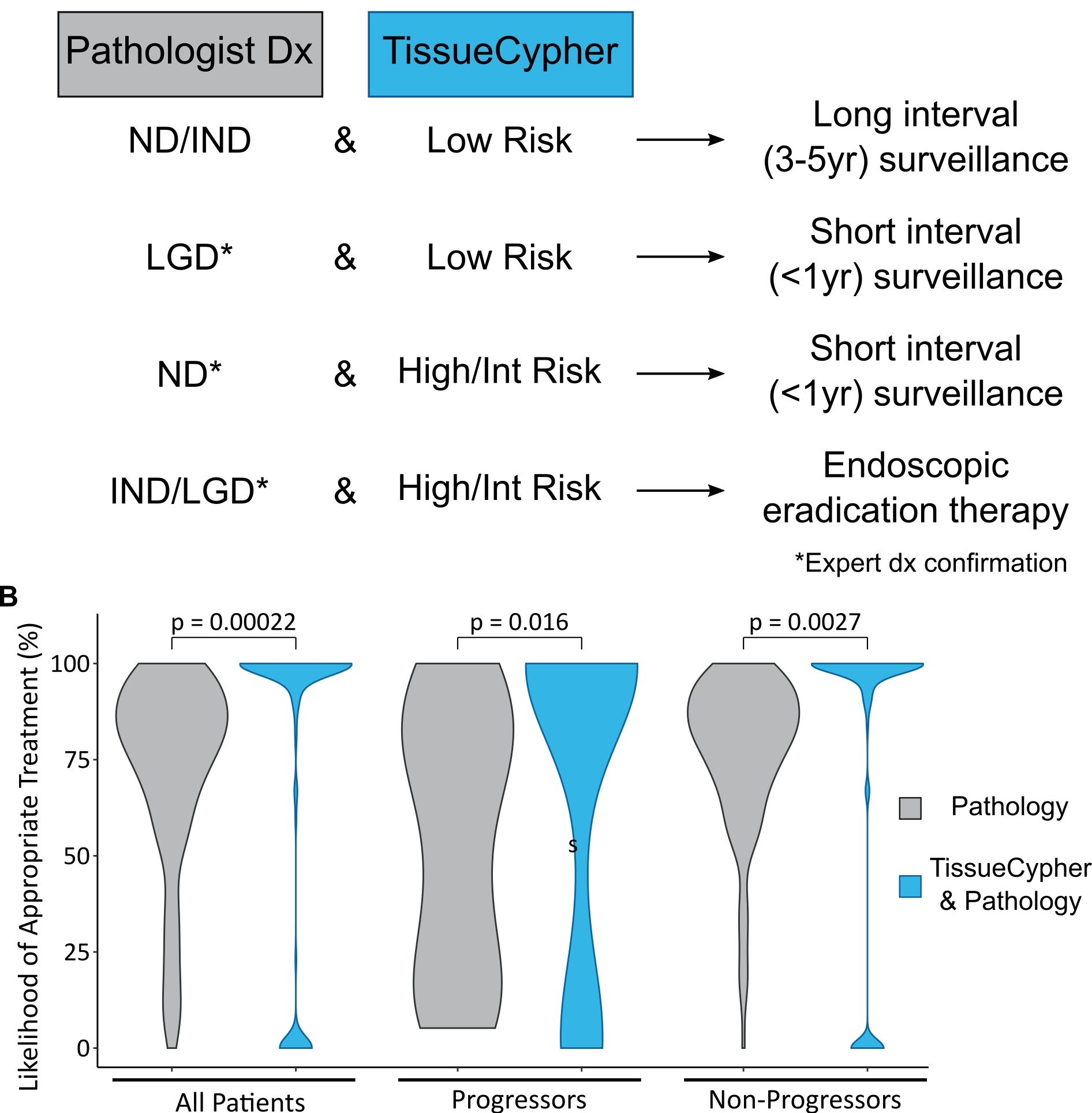
Methods: 154 BE patients with community-based LGD from the prospectively-followed screening cohort of the SURF trial were studied. All biopsies from the baseline endoscopy were independently reviewed by 16 generalist and 14 expert pathologists from five countries, and also assessed by the spatialomics test that scores patients as low-, intermediate or high-risk for progression to HGD/EAC. Each patient’s journey from diagnosis to surveillance and treatment was simulated 500 times with varying pathology reviewers to determine the most likely care plan with or without the test to guide management (Figure 1A). The percentage of patients receiving appropriate management was calculated based on known progression/non-progression outcomes.
Results: Use of the spatialomics test in conjunction with pathology review of LGD increased the likelihood of appropriate management from median 80% (IQR 63-91) to 100% (89-100) (p=0.00022). Likelihood of appropriate management was significantly increased in the patients who progressed to HGD/EAC from median 63% (IQR 24-83) to 100% (IQR 23-100) (p=0.016) and from 82% (IQR 70-93) to 100% (IQR 91-100) (p=0.0027) for patients that did not progress within 10 years (Figure 1B).
Discussion: The spatialomics test offers an effective solution to subjective and variable pathology review by providing objective risk stratification in BE patients with a community-based diagnosis of LGD. Management guided by this test may help to standardize care plans, increasing the early detection of progressor patients who can receive interventions that effectively prevent progression, while also increasing the percentage of non-progressors who can safely avoid unnecessary therapy and be managed by a surveillance-only approach.
Disclosures:
Nicola F. F. Frei, MD1, Lucas C. Duits, MD, PhD2, Amir Khoshiwal, MD3, Roos E. Pouw, MD, PhD3, Christian Smolko, PhD4, Meenakshi Arora, PhD4, Jennifer J. Siegel, MA, PhD5, Rebecca Critchley-Thorne, PhD4, Jacques Bergman, MD, PhD6, 10, An Objective Spatialomics Test Standardizes Management Decisions With Potential to Improve Health Outcomes for Barrett’s Esophagus Patients, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Nicola F. F. Frei, MD1, Lucas C. Duits, MD, PhD2, Amir Khoshiwal, MD3, Roos E. Pouw, MD, PhD3, Christian Smolko, PhD4, Meenakshi Arora, PhD4, Jennifer J. Siegel, MA, PhD5, Rebecca Critchley-Thorne, PhD4, Jacques Bergman, MD, PhD6
1University Medical Center Amsterdam, Amsterdam, Noord-Holland, Netherlands; 2Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, Noord-Holland, Netherlands; 3Amsterdam UMC, Amsterdam, Noord-Holland, Netherlands; 4Castle Biosciences, Pittsburgh, PA; 5Castle Biosciences, Friendswood, TX; 6Amsterdam University Medical Centres, Amsterdam, Noord-Holland, Netherlands
Introduction: The diagnosis of low-grade dysplasia (LGD) in Barrett’s esophagus (BE) is associated with increased risk of progression to high grade dysplasia (HGD) or esophageal adenocarcinoma (EAC). However, due to substantial observer variability in the diagnosis of LGD, a patient’s management plan and health outcome depends largely on which pathologist reviews their case. Recent studies have shown that an objective spatialomics test (TissueCypher) predicts neoplastic progression in BE patients with higher sensitivity than pathology review. This study evaluated the test’s potential to augment pathology to standardize clinical management in a manner consistent with improved health outcomes for BE patients.
Methods: 154 BE patients with community-based LGD from the prospectively-followed screening cohort of the SURF trial were studied. All biopsies from the baseline endoscopy were independently reviewed by 16 generalist and 14 expert pathologists from five countries, and also assessed by the spatialomics test that scores patients as low-, intermediate or high-risk for progression to HGD/EAC. Each patient’s journey from diagnosis to surveillance and treatment was simulated 500 times with varying pathology reviewers to determine the most likely care plan with or without the test to guide management (Figure 1A). The percentage of patients receiving appropriate management was calculated based on known progression/non-progression outcomes.
Results: Use of the spatialomics test in conjunction with pathology review of LGD increased the likelihood of appropriate management from median 80% (IQR 63-91) to 100% (89-100) (p=0.00022). Likelihood of appropriate management was significantly increased in the patients who progressed to HGD/EAC from median 63% (IQR 24-83) to 100% (IQR 23-100) (p=0.016) and from 82% (IQR 70-93) to 100% (IQR 91-100) (p=0.0027) for patients that did not progress within 10 years (Figure 1B).
Discussion: The spatialomics test offers an effective solution to subjective and variable pathology review by providing objective risk stratification in BE patients with a community-based diagnosis of LGD. Management guided by this test may help to standardize care plans, increasing the early detection of progressor patients who can receive interventions that effectively prevent progression, while also increasing the percentage of non-progressors who can safely avoid unnecessary therapy and be managed by a surveillance-only approach.

Figure: Figure 1. TissueCypher-guided management increases the percentage of BE patients with community-based diagnosis of LGD who receive appropriate management with the potential to improve outcomes. A. TissueCypher-guided management in conjunction with pathology review of patients with initial community-based diagnosis of LGD; B. Likelihood of appropriate management using pathology review alone vs TissueCypher-guided management in conjunction with pathology. Appropriate management for progressors was endoscopic eradication therapy or short interval surveillance in less than 1 year. Appropriate management for non-progressors was long interval surveillance in 3-5 years. The pathology alone arm assumed that clinicians followed the current standard of care guidelines. Maximum width of violin plots held constant; within each paired group (All, Progressors, Non-Progressors) observation number is the same. p values calculated by Wilcoxon paired test.
Disclosures:
Nicola F. Frei indicated no relevant financial relationships.
Lucas Duits indicated no relevant financial relationships.
Amir Khoshiwal indicated no relevant financial relationships.
Roos Pouw: Medtronic – Consultant. MicroTech Endoscopy – Consultant. Pentax – Speaker fee.
Christian Smolko: Castle Biosciences – Employee. Noveome Biotherapeutics – Employee.
Meenakshi Arora indicated no relevant financial relationships.
Jennifer Siegel: Castle Biosciences – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rebecca Critchley-Thorne: Castle Biosciences – Employee.
Jacques Bergman: Castle Science – Grant/Research Support.
Nicola F. F. Frei, MD1, Lucas C. Duits, MD, PhD2, Amir Khoshiwal, MD3, Roos E. Pouw, MD, PhD3, Christian Smolko, PhD4, Meenakshi Arora, PhD4, Jennifer J. Siegel, MA, PhD5, Rebecca Critchley-Thorne, PhD4, Jacques Bergman, MD, PhD6, 10, An Objective Spatialomics Test Standardizes Management Decisions With Potential to Improve Health Outcomes for Barrett’s Esophagus Patients, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.


