Back


Poster Session A - Sunday Afternoon
Category: GI Bleeding
A0298 - Efficacy of Thalidomide for the Treatment of Gastrointestinal Bleeding From Vascular Malformation: A Meta-Analysis and Systematic Review
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Yuntao Zou, MD
Saint Peter's University Hospital
New Brunswick, NJ
Presenting Author(s)
Yuntao Zou, MD1, Nan Gao, MD2, Mohamed Abdelbaky, MD3, Dhruv Singh, MBBS1, Yi-Chia Wu, MD4, Bing Chen, MD5, Yichen Wang, MD6, Howard Chung, MD7, Arkady Broder, MD, FACG4
1Saint Peter's University Hospital, New Brunswick, NJ; 2Lincoln Medical Center, Bronx, NY; 3Rutgers Robert Wood Johnson Medical School/Saint Peter's University Hospital, New Brunswick, NJ; 4Rutgers Medical School of Robert Wood Johnson, Saint Peter's University Hospital, New Brunswick, NJ; 5New York University School of Medicine, New York, NY; 6Trinity Health of New England, Springfield, MA; 7New York University School of Medicine, Brooklyn, NY
Introduction: Gastrointestinal bleeding from vascular malformation is hard to treat. Thalidomide has been shown with therapeutic effects in several studies. We performed a meta-analysis for its efficacy on GI bleeding due to vascular malformation.
Methods: MEDLINE, the Cochrane Library, and EMBASE were searched up to June 5th. The following keywords were used: “Arteriovenous Malformation”, “AVM”, “Angioectasia”, “Angiodysplasia”, “Vascular Malformation”, “Telangiectasia”, "Thalidomide", "Contergan", "Thalomid", "α-Phthalimidoglutarimide". Observational studies and clinical trials that utilized Nivolumab for refractory esophageal cancer were included. Bleeding cessation rates were studied as primary outcomes. Data were analyzed with STATA version 16.0 (Stata Corp, College Station, TX, USA).
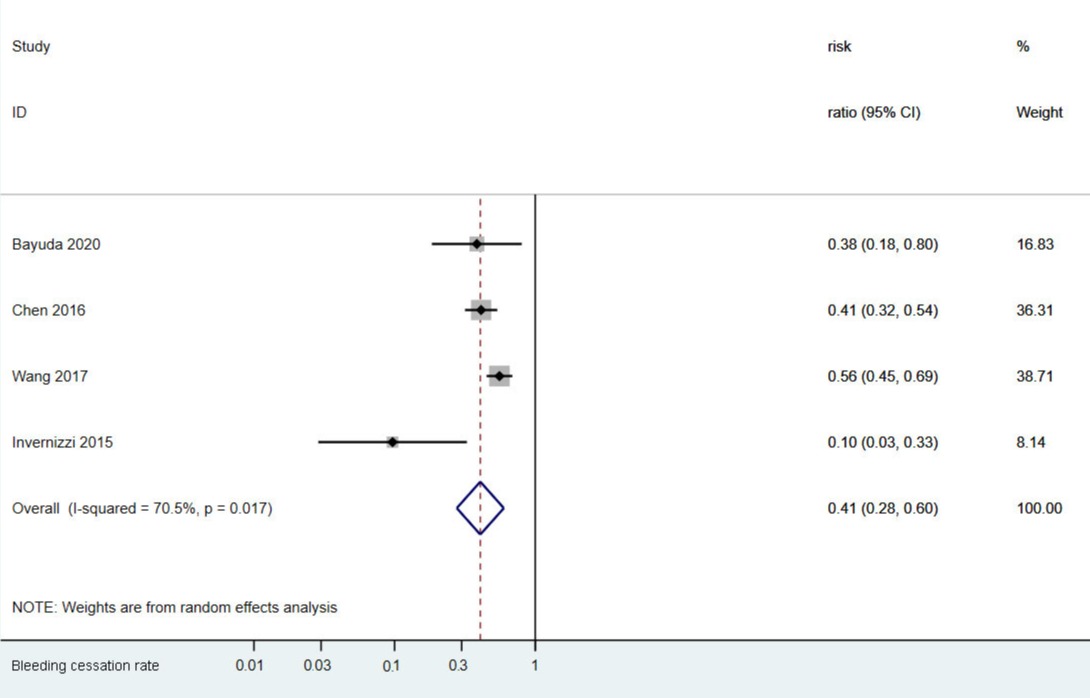
Results: A total of 405 manuscripts were identified and four observational or clinical studies with 194 patients meeting inclusion criteria. Patient median or mean ages were more than 50 in all 4 studies and 89 (45.4%) individuals were male. The dose of thalidomide ranged from 50 mg to 200 mg per day. The duration was from 3 months to 45 months. For patients with gastrointestinal bleeding from vascular malformation, thalidomide has a bleeding cessation rate of 41% (95%, 28%-60%) in 6-12 months.
Discussion: Many of the studies claimed that thalidomide was able to decrease bleeding cessation rates significantly, while our meta-analysis with all available studies did not show a significant decrease in bleeding cessation rates compared to the non-thalidomide group reported by Wang’s study (41% vs 46%) (Fig.1). Several studies showed that thalidomide was helpful in the yearly bleeding episodes, yearly red blood cell transfusion requirement, transfusion dependence, overall and bleeding-related hospitalization rate, endoscopic treatment requirement, and hemoglobin level changes, but none of the above topics had enough data to perform a meta-analysis. Therefore, further studies are needed to evaluate the efficacy of thalidomide on Gastrointestinal bleeding from vascular malformation, besides the bleeding cessation rates.
Disclosures:
Yuntao Zou, MD1, Nan Gao, MD2, Mohamed Abdelbaky, MD3, Dhruv Singh, MBBS1, Yi-Chia Wu, MD4, Bing Chen, MD5, Yichen Wang, MD6, Howard Chung, MD7, Arkady Broder, MD, FACG4. A0298 - Efficacy of Thalidomide for the Treatment of Gastrointestinal Bleeding From Vascular Malformation: A Meta-Analysis and Systematic Review, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Saint Peter's University Hospital, New Brunswick, NJ; 2Lincoln Medical Center, Bronx, NY; 3Rutgers Robert Wood Johnson Medical School/Saint Peter's University Hospital, New Brunswick, NJ; 4Rutgers Medical School of Robert Wood Johnson, Saint Peter's University Hospital, New Brunswick, NJ; 5New York University School of Medicine, New York, NY; 6Trinity Health of New England, Springfield, MA; 7New York University School of Medicine, Brooklyn, NY
Introduction: Gastrointestinal bleeding from vascular malformation is hard to treat. Thalidomide has been shown with therapeutic effects in several studies. We performed a meta-analysis for its efficacy on GI bleeding due to vascular malformation.
Methods: MEDLINE, the Cochrane Library, and EMBASE were searched up to June 5th. The following keywords were used: “Arteriovenous Malformation”, “AVM”, “Angioectasia”, “Angiodysplasia”, “Vascular Malformation”, “Telangiectasia”, "Thalidomide", "Contergan", "Thalomid", "α-Phthalimidoglutarimide". Observational studies and clinical trials that utilized Nivolumab for refractory esophageal cancer were included. Bleeding cessation rates were studied as primary outcomes. Data were analyzed with STATA version 16.0 (Stata Corp, College Station, TX, USA).
Results: A total of 405 manuscripts were identified and four observational or clinical studies with 194 patients meeting inclusion criteria. Patient median or mean ages were more than 50 in all 4 studies and 89 (45.4%) individuals were male. The dose of thalidomide ranged from 50 mg to 200 mg per day. The duration was from 3 months to 45 months. For patients with gastrointestinal bleeding from vascular malformation, thalidomide has a bleeding cessation rate of 41% (95%, 28%-60%) in 6-12 months.
Discussion: Many of the studies claimed that thalidomide was able to decrease bleeding cessation rates significantly, while our meta-analysis with all available studies did not show a significant decrease in bleeding cessation rates compared to the non-thalidomide group reported by Wang’s study (41% vs 46%) (Fig.1). Several studies showed that thalidomide was helpful in the yearly bleeding episodes, yearly red blood cell transfusion requirement, transfusion dependence, overall and bleeding-related hospitalization rate, endoscopic treatment requirement, and hemoglobin level changes, but none of the above topics had enough data to perform a meta-analysis. Therefore, further studies are needed to evaluate the efficacy of thalidomide on Gastrointestinal bleeding from vascular malformation, besides the bleeding cessation rates.

Figure: Forest plot of thalidomide’s effect on cessation of gastrointestinal bleeding from vascular malformation.
Disclosures:
Yuntao Zou indicated no relevant financial relationships.
Nan Gao indicated no relevant financial relationships.
Mohamed Abdelbaky indicated no relevant financial relationships.
Dhruv Singh indicated no relevant financial relationships.
Yi-Chia Wu indicated no relevant financial relationships.
Bing Chen indicated no relevant financial relationships.
Yichen Wang indicated no relevant financial relationships.
Howard Chung indicated no relevant financial relationships.
Arkady Broder: GITrak – Advisory Committee/Board Member.
Yuntao Zou, MD1, Nan Gao, MD2, Mohamed Abdelbaky, MD3, Dhruv Singh, MBBS1, Yi-Chia Wu, MD4, Bing Chen, MD5, Yichen Wang, MD6, Howard Chung, MD7, Arkady Broder, MD, FACG4. A0298 - Efficacy of Thalidomide for the Treatment of Gastrointestinal Bleeding From Vascular Malformation: A Meta-Analysis and Systematic Review, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
