Back

Poster Session A - Sunday Afternoon
Category: Colon
A0140 - Sevelamer-Induced Colitis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Karthik Mathialagan, MD
Bridgeport Hospital, Yale New Haven Health
Trumbull, CT
Presenting Author(s)
Karthik Mathialagan, MD, Mark S. McFarland, MD, MSc, Patricia Garcia, MD, Caroline Loeser, MD
Bridgeport Hospital, Yale New Haven Health, Bridgeport, CT
Introduction: Drug-induced Gastrointestinal (GI) injury has been well established throughout the literature and continues to prevail with the ever-growing expansion of pharmaceutical drug development. Mucosal injuries due to medications ranging from common over-the-counter drugs to novel chemotherapeutics, have been one of the more common findings seen both endoscopically and histologically in drug-induced GI injury. Though frequently seen with NSAIDs, iron pills, and bisphosphonates, lately, resin-based binders like Sevelamer have shown an increased incidence of GI mucosal injuries. Sevelamer is an oral phosphate binder widely used to treat hyperphosphatemia in patients with chronic kidney disease. Here we present a case of Sevelamer induced colitis in a patient on Sevelamer Carbonate therapy for hyperphosphatemia secondary to acute kidney injury.
Case Description/Methods: 34 year old male with history of hypertension, obesity class III status post sleeve gastrectomy, OSA, congestive heart failure, and a recent diagnosis of COVID-19 was admitted to the MICU for acute hypoxemic respiratory failure requiring intubation. His hospital course was complicated by worsening renal function and oliguria with creatinine 8.9 and phosphorus 6.4. He was started on hemodialysis (HD), followed by Sevelamer Carbonate 0.8g TID then increased to 1.6g TID. Weeks after initiating HD, he developed bright red blood per rectum requiring intermittent transfusions. CT abdomen pelvis and mesenteric angiogram were unremarkable. Upper GI endoscopy showed a Forest Class III duodenal ulcer. Colonoscopy showed localized severe friable ulcerated mucosa with loss of vascular pattern in the cecum and ascending colon. Biopsy at these sites revealed mucosal ulcerations with granulation tissue with Sevelamer crystal deposits. Hematochezia improved on discontinuing Sevelamer.
Discussion: Sevelamer is a calcium-free oral phosphate binder that has replaced calcium-based binders to treat hyperphosphatemia. Common side effects include nausea, vomiting, and diarrhea. GI mucosal injury from resin-based binders has been reported, although colitis induced by Sevelamer crystal deposition is rare. Previous literature shows a wide range of presentations, including ulcerations, GI bleeding, and colonic strictures. As with prior reports, we show similar endoscopic and histological findings from our patient consistent with this rare entity.
Disclosures:
Karthik Mathialagan, MD, Mark S. McFarland, MD, MSc, Patricia Garcia, MD, Caroline Loeser, MD. A0140 - Sevelamer-Induced Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Bridgeport Hospital, Yale New Haven Health, Bridgeport, CT
Introduction: Drug-induced Gastrointestinal (GI) injury has been well established throughout the literature and continues to prevail with the ever-growing expansion of pharmaceutical drug development. Mucosal injuries due to medications ranging from common over-the-counter drugs to novel chemotherapeutics, have been one of the more common findings seen both endoscopically and histologically in drug-induced GI injury. Though frequently seen with NSAIDs, iron pills, and bisphosphonates, lately, resin-based binders like Sevelamer have shown an increased incidence of GI mucosal injuries. Sevelamer is an oral phosphate binder widely used to treat hyperphosphatemia in patients with chronic kidney disease. Here we present a case of Sevelamer induced colitis in a patient on Sevelamer Carbonate therapy for hyperphosphatemia secondary to acute kidney injury.
Case Description/Methods: 34 year old male with history of hypertension, obesity class III status post sleeve gastrectomy, OSA, congestive heart failure, and a recent diagnosis of COVID-19 was admitted to the MICU for acute hypoxemic respiratory failure requiring intubation. His hospital course was complicated by worsening renal function and oliguria with creatinine 8.9 and phosphorus 6.4. He was started on hemodialysis (HD), followed by Sevelamer Carbonate 0.8g TID then increased to 1.6g TID. Weeks after initiating HD, he developed bright red blood per rectum requiring intermittent transfusions. CT abdomen pelvis and mesenteric angiogram were unremarkable. Upper GI endoscopy showed a Forest Class III duodenal ulcer. Colonoscopy showed localized severe friable ulcerated mucosa with loss of vascular pattern in the cecum and ascending colon. Biopsy at these sites revealed mucosal ulcerations with granulation tissue with Sevelamer crystal deposits. Hematochezia improved on discontinuing Sevelamer.
Discussion: Sevelamer is a calcium-free oral phosphate binder that has replaced calcium-based binders to treat hyperphosphatemia. Common side effects include nausea, vomiting, and diarrhea. GI mucosal injury from resin-based binders has been reported, although colitis induced by Sevelamer crystal deposition is rare. Previous literature shows a wide range of presentations, including ulcerations, GI bleeding, and colonic strictures. As with prior reports, we show similar endoscopic and histological findings from our patient consistent with this rare entity.

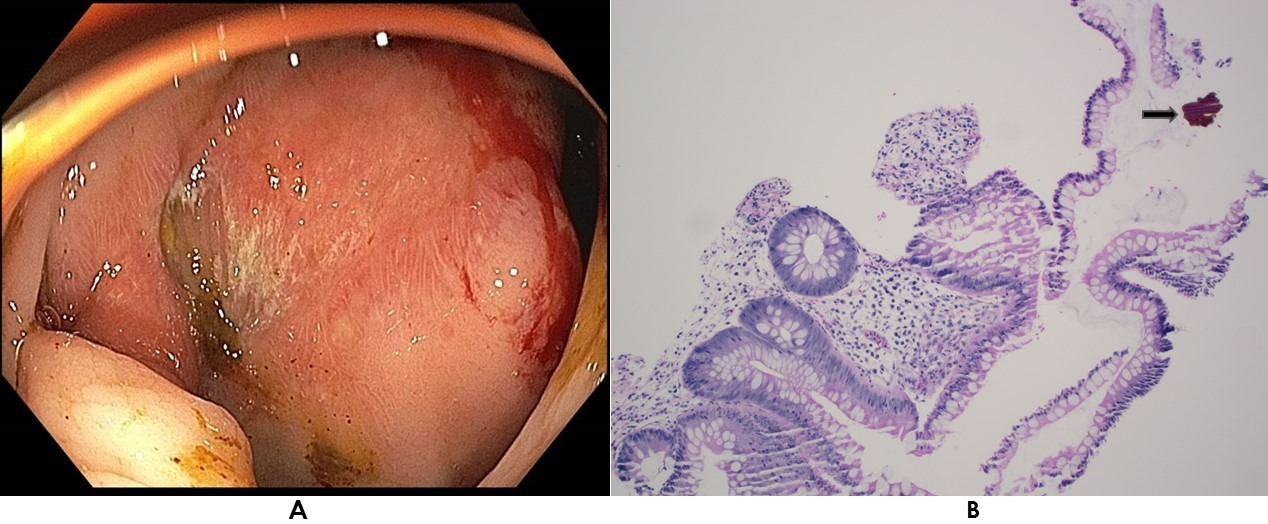
Figure: Figures: A - Colonoscopic finding showing erythematous ulcerated mucosa in the proximal ascending colon.
B - Biopsy from proximal ascending colon site showing granulation tissue with ulceration. The black arrow indicates the Sevelamer Crystal deposition, showing the characteristic “Fish Scale” pattern.
B - Biopsy from proximal ascending colon site showing granulation tissue with ulceration. The black arrow indicates the Sevelamer Crystal deposition, showing the characteristic “Fish Scale” pattern.
Disclosures:
Karthik Mathialagan indicated no relevant financial relationships.
Mark McFarland indicated no relevant financial relationships.
Patricia Garcia indicated no relevant financial relationships.
Caroline Loeser indicated no relevant financial relationships.
Karthik Mathialagan, MD, Mark S. McFarland, MD, MSc, Patricia Garcia, MD, Caroline Loeser, MD. A0140 - Sevelamer-Induced Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
