Back


Poster Session C - Monday Afternoon
Category: Colorectal Cancer Prevention
C0175 - A Single Centered Retrospective Review of Colonoscopy Results in Patients With Positive Cologuard
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Muhammad Sajeel Anwar, MD
United Health Services
Vestal, NY
Presenting Author(s)
Muhammad Sajeel Anwar, MD1, Mohammad Ali Abidi, 2, Leslie Bank, MBChB, MD, FACG3
1United Health Services, Vestal, NY; 2United Health Services, Johnson City, NY; 3United Health Services, Binghamton, NY
Introduction: Colorectal cancer (CRC) is the most common form of gastrointestinal cancer and is the third leading cause of cancer deaths in the US. At this time, effective screening tools used for colon adenomas and cancer detection are fecal occult blood test (FOBT), fecal immunochemical tests (FIT), stool DNA test (Cologuard), and optical colonoscopy. A limitation of FOBT and FIT tests is that they carry low positive predictive values. Cologuard is indicated in colorectal cancer screening in “average risk” adults performed in three-year intervals. Currently, Cologuard is considered 92 % sensitive and 87% specific in colon cancer detection in the average-risk population. In our retrospective study, we will be comparing the results of positive Cologuard with subsequent colonoscopy findings.
Methods:
Results:
Discussion:
Disclosures:
Muhammad Sajeel Anwar, MD1, Mohammad Ali Abidi, 2, Leslie Bank, MBChB, MD, FACG3. C0175 - A Single Centered Retrospective Review of Colonoscopy Results in Patients With Positive Cologuard, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1United Health Services, Vestal, NY; 2United Health Services, Johnson City, NY; 3United Health Services, Binghamton, NY
Introduction: Colorectal cancer (CRC) is the most common form of gastrointestinal cancer and is the third leading cause of cancer deaths in the US. At this time, effective screening tools used for colon adenomas and cancer detection are fecal occult blood test (FOBT), fecal immunochemical tests (FIT), stool DNA test (Cologuard), and optical colonoscopy. A limitation of FOBT and FIT tests is that they carry low positive predictive values. Cologuard is indicated in colorectal cancer screening in “average risk” adults performed in three-year intervals. Currently, Cologuard is considered 92 % sensitive and 87% specific in colon cancer detection in the average-risk population. In our retrospective study, we will be comparing the results of positive Cologuard with subsequent colonoscopy findings.
Methods:
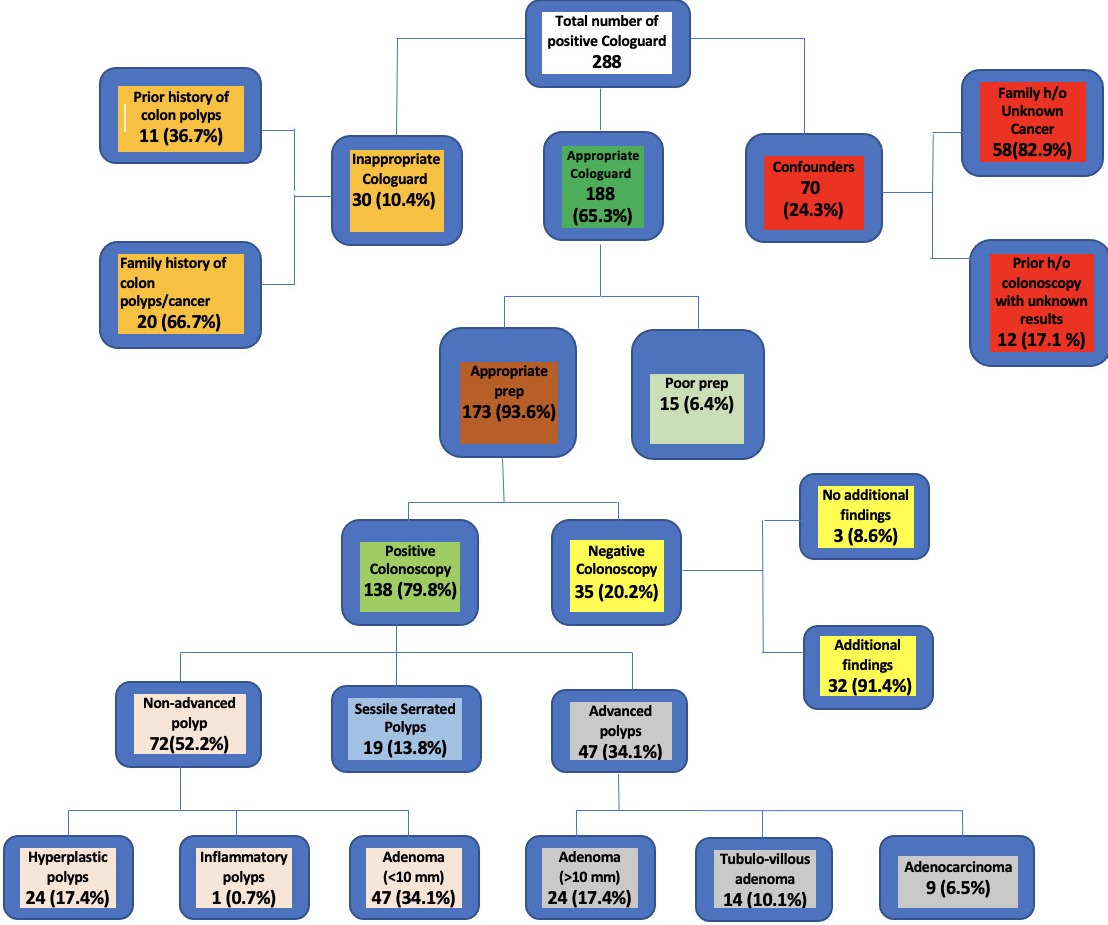
- 288 patients with positive Cologuard test were reviewed and compared with results of follow-up colonoscopies.
- The relevant information was transcribed into an excel file. Information including, but not limited to, MRN, Age, Height, Weight, BMI, Medication, Family History, results and date of positive Cologuard, results, and follow-up colonoscopies, was assessed.
- Patients were identified by utilizing their medical record numbers through the ProVation software. Prior to analysis, de-identification of patient’s data was performed.
Results:
- Out of 288 patients who tested positive on Cologuard screening,
- 10.4% of patients (30/288) were excluded as they were noted to have undergone an inappropriate Cologuard test.
- 24.3% of patients (70/288) were excluded because of a family history of unknown cancer (58/70, 82.9%) and prior history of colonoscopy with unknown results (12/70, 17.1%).
- 6.4% (15/188) were excluded because of poor preparation.
- 93.6% of patients (173/188) had appropriate preparation. Of these,
- 20.2% (35/176) of patients had negative colonoscopies for polyps/CRC.
- 79.8% (138/176) had positive colonoscopies. Of these patients, 52.2% (72/138) had non-advanced polyps, 13.8% (19/138) had sessile serrated polyps and 34.1% (47/138) had advanced polyps.
Discussion:
- In our patient population, 10.7% (30/288) patients underwent inappropriate Cologuard testing.
- 65.3% (188/288) patients underwent appropriate Cologuard testing with:
- 6.4% (12/188) of patients were removed because of poor prep.
- 20.2% (35/176) of patients had a normal colonoscopy.
- 79.8% (138/176) patients resulted in an abnormal colonoscopy with 6.5% (9/138) patients diagnosed with colorectal cancer.

Figure: Flowsheet.
Disclosures:
Muhammad Sajeel Anwar indicated no relevant financial relationships.
Mohammad Ali Abidi indicated no relevant financial relationships.
Leslie Bank indicated no relevant financial relationships.
Muhammad Sajeel Anwar, MD1, Mohammad Ali Abidi, 2, Leslie Bank, MBChB, MD, FACG3. C0175 - A Single Centered Retrospective Review of Colonoscopy Results in Patients With Positive Cologuard, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
