Back

Poster Session A - Sunday Afternoon
Category: GI Bleeding
A0304 - A Rare Case of SpaceOARTM Hydrogel-Induced Rectal Ulcer Leading to Hematochezia
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Janak Bahirwani, MD
St. Luke's University Health Network
Bethlehem, PA
Presenting Author(s)
Janak Bahirwani, MD, Brittney Shupp, DO, Ronak Modi, MD
St. Luke's University Health Network, Bethlehem, PA
Introduction: SpaceOAR hydrogel is a soft gel-like synthetic material that was FDA approved in 2015 for prostate cancer patients who are planning to undergo radiotherapy (RT). It is intended to position the anterior rectal wall away from the prostate during RT thereby reducing the dose of radiation delivered to the rectum and minimizing complications. We present a case of rectal ulcer after implantation of SpaceOAR prior to the patient receiving RT.
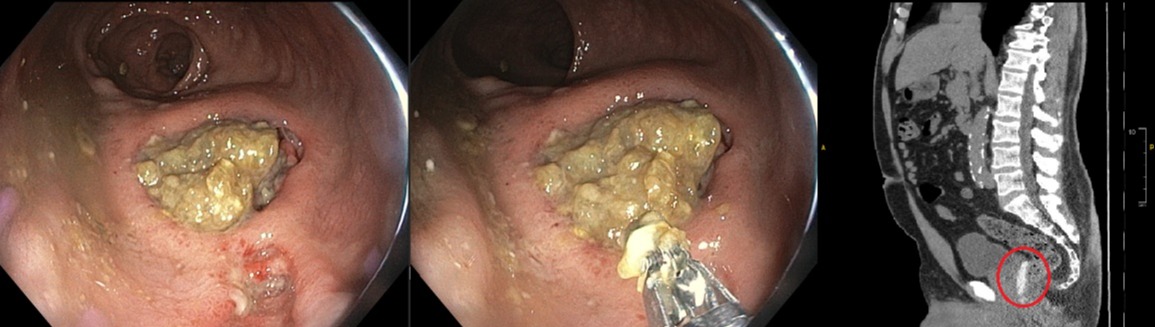
Case Description/Methods: A 76-year-old male was diagnosed with prostate cancer- Gleason 3+4 and was planned for RT. He underwent implantation of spaceOAR hydrogel and gold fiducial markers before RT to minimize his risk of rectal toxicity. Approximately 10 days after placement, he started experiencing severe rectal pain and hematochezia. On physical exam his abdomen was benign, but a digital rectal exam showed bright red blood. Labs revealed normal hemoglobin of 16 g/dL. A computerized tomography (CT) scan without contrast was obtained showing the presence of hydrogel between the posterior margin of the prostate and the anterior wall of the low rectum with focal proctitis in that area of the rectum. Due to ongoing hematochezia, he was scheduled for a colonoscopy which confirmed CT findings of focal proctitis but also showed a large ulcer with overlying fibrotic tissue congruent with the site of his recent urological procedure. The tissue was friable and not amenable to biopsy. He was managed with a course of levofloxacin and given polyethylene glycol to avoid constipation. At a follow-up visit, he reported resolution of hematochezia and improvement in his rectal pain. He will proceed with the initiation of RT for prostate cancer.
Discussion: The SpaceOAR Hydrogel is designed to reduce rectal toxicity during RT for prostate cancer so a higher dose of radiation can be delivered to the prostate. There have been only a few cases of rectal ulcers after placement of the Hydrogel before the patient received RT. In most reported cases, the management was conservative. The mechanisms of injury are thought to be from infection, inflammation, or ischemia due to mechanical injury. We managed our patient with a course of antibiotics and laxatives resulting in improvement of his symptoms and he will proceed with RT as planned. This case is unique in that the patient developed symptoms only 10 days after implantation of SpaceOAR when all other documented cases took at least a few weeks to a few months.
Disclosures:
Janak Bahirwani, MD, Brittney Shupp, DO, Ronak Modi, MD. A0304 - A Rare Case of SpaceOARTM Hydrogel-Induced Rectal Ulcer Leading to Hematochezia, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
St. Luke's University Health Network, Bethlehem, PA
Introduction: SpaceOAR hydrogel is a soft gel-like synthetic material that was FDA approved in 2015 for prostate cancer patients who are planning to undergo radiotherapy (RT). It is intended to position the anterior rectal wall away from the prostate during RT thereby reducing the dose of radiation delivered to the rectum and minimizing complications. We present a case of rectal ulcer after implantation of SpaceOAR prior to the patient receiving RT.
Case Description/Methods: A 76-year-old male was diagnosed with prostate cancer- Gleason 3+4 and was planned for RT. He underwent implantation of spaceOAR hydrogel and gold fiducial markers before RT to minimize his risk of rectal toxicity. Approximately 10 days after placement, he started experiencing severe rectal pain and hematochezia. On physical exam his abdomen was benign, but a digital rectal exam showed bright red blood. Labs revealed normal hemoglobin of 16 g/dL. A computerized tomography (CT) scan without contrast was obtained showing the presence of hydrogel between the posterior margin of the prostate and the anterior wall of the low rectum with focal proctitis in that area of the rectum. Due to ongoing hematochezia, he was scheduled for a colonoscopy which confirmed CT findings of focal proctitis but also showed a large ulcer with overlying fibrotic tissue congruent with the site of his recent urological procedure. The tissue was friable and not amenable to biopsy. He was managed with a course of levofloxacin and given polyethylene glycol to avoid constipation. At a follow-up visit, he reported resolution of hematochezia and improvement in his rectal pain. He will proceed with the initiation of RT for prostate cancer.
Discussion: The SpaceOAR Hydrogel is designed to reduce rectal toxicity during RT for prostate cancer so a higher dose of radiation can be delivered to the prostate. There have been only a few cases of rectal ulcers after placement of the Hydrogel before the patient received RT. In most reported cases, the management was conservative. The mechanisms of injury are thought to be from infection, inflammation, or ischemia due to mechanical injury. We managed our patient with a course of antibiotics and laxatives resulting in improvement of his symptoms and he will proceed with RT as planned. This case is unique in that the patient developed symptoms only 10 days after implantation of SpaceOAR when all other documented cases took at least a few weeks to a few months.

Figure: A- Focal proctitis and large rectal ulcer in the anterior wall
B- Friable tissue not amenable to biopsy
C- CT scan showing hydrogel and focal proctitis
B- Friable tissue not amenable to biopsy
C- CT scan showing hydrogel and focal proctitis
Disclosures:
Janak Bahirwani indicated no relevant financial relationships.
Brittney Shupp indicated no relevant financial relationships.
Ronak Modi indicated no relevant financial relationships.
Janak Bahirwani, MD, Brittney Shupp, DO, Ronak Modi, MD. A0304 - A Rare Case of SpaceOARTM Hydrogel-Induced Rectal Ulcer Leading to Hematochezia, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
