Back


Poster Session C - Monday Afternoon
Category: Liver
C0587 - Ashwagandha Toxicity: A Rare Case of Drug Induced Liver Injury (DILI)
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

James Gnecco, DO
University of Texas Health Science Center
San Antonio, TX
Presenting Author(s)
James Gnecco, DO1, Hasan Baher, MD1, Brenda M. Briones, MD1, Fred Poordad, MD2
1University of Texas Health Science Center, San Antonio, TX; 2UTHSCSA, San Antonio, TX
Introduction: The pursuance of natural and herbal remedies by many has brought with
it further knowledge of the many causes of Drug Induced Liver Injury (DILI). Many
of these "natural" over-the-counter (OTC) supplements may not be benign due to the
lack of scientific analysis and unregulated production. Ashwagandha is a supplement
used for memory enhancement, management of anxiety, and general increase in
vitality. We present a case of Ashwagandha DILI in a healthy 36-year-old man.
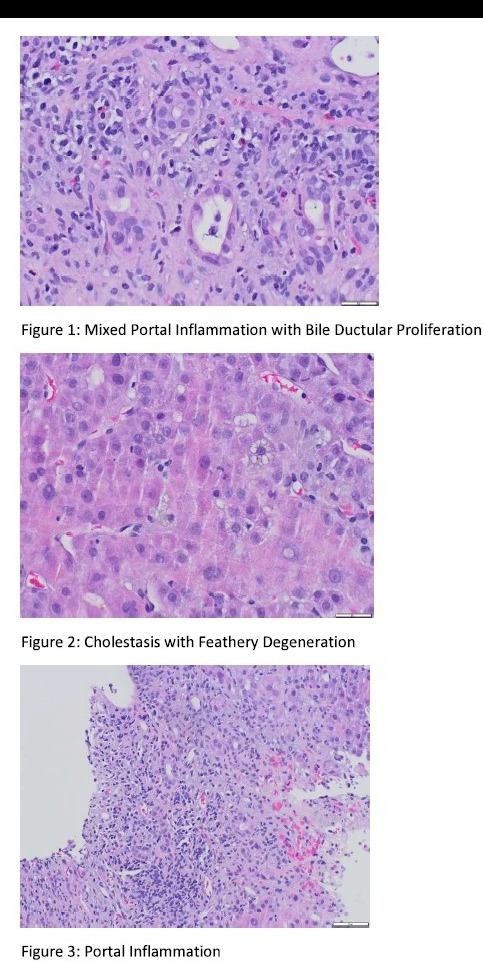
Case Description/Methods: A 36-year-old male presented to our institution with 1 week of fatigue, jaundice, nausea and subjective fevers. Upon presentation he was hemodynamically stable and afebrile. Physical exam was notable for jaundice without other stigmata of liver disease or mental status changes. He was found to have acute liver injury with lab chemistries remarkable for AST= 1482 U/L, ALT= 1375 U/L, Tbili= 22.3 mg/dL, alkaline phosphatase= 202 U/L and an INR of 1.6. He denied alcohol and drug use, recent travel or sick contacts. Medications list was notable for cetirizine, diphenhydramine (both taken as needed), OTC testosterone supplements, OTC apple cider vinegar gummies BID and OTC ashwagandha gummies BID. Workup was negative for autoimmune, infectious, metabolic, obstructive and vascular causes of liver injury. Acetaminophen level and urine drug screen were negative. Trans-jugular liver biopsy revealed evidence of acute portal and lobular hepatitis and cholestasis, suggestive of DILI. Patient's liver enzymes continued to downtrend (AST=929, ALT=765, Tbili=22.5) after withdrawal of his supplements and was discharged.
Discussion: Ashwagandha causing DILI has been infrequently reported in the
literature. One case series of 5 patients in Iceland and a single case in Japan reported
that ashwagandha is an uncommon culprit linked to acute liver injury. None of the
reported cases necessitated liver transplant. Average time to resolution and
normalization of LFTs was 3.5 months and R values usually ranged in the mixed
range (R value 2-5). The case series also revealed that ashwagandha's toxicity could
be dose dependent, as higher peaks in LFT's were tied to recent increases in
ashwagandha dosing. Although ashwagandha has been a rarely reported cause of
DILI, a thorough history of medication and OTC supplements in patients with
undifferentiated acute liver injury may yield more cases. Further study and reporting
of DILI in the ever-growing supplement market appears to be required to minimize
potential injury.
Disclosures:
James Gnecco, DO1, Hasan Baher, MD1, Brenda M. Briones, MD1, Fred Poordad, MD2. C0587 - Ashwagandha Toxicity: A Rare Case of Drug Induced Liver Injury (DILI), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Texas Health Science Center, San Antonio, TX; 2UTHSCSA, San Antonio, TX
Introduction: The pursuance of natural and herbal remedies by many has brought with
it further knowledge of the many causes of Drug Induced Liver Injury (DILI). Many
of these "natural" over-the-counter (OTC) supplements may not be benign due to the
lack of scientific analysis and unregulated production. Ashwagandha is a supplement
used for memory enhancement, management of anxiety, and general increase in
vitality. We present a case of Ashwagandha DILI in a healthy 36-year-old man.
Case Description/Methods: A 36-year-old male presented to our institution with 1 week of fatigue, jaundice, nausea and subjective fevers. Upon presentation he was hemodynamically stable and afebrile. Physical exam was notable for jaundice without other stigmata of liver disease or mental status changes. He was found to have acute liver injury with lab chemistries remarkable for AST= 1482 U/L, ALT= 1375 U/L, Tbili= 22.3 mg/dL, alkaline phosphatase= 202 U/L and an INR of 1.6. He denied alcohol and drug use, recent travel or sick contacts. Medications list was notable for cetirizine, diphenhydramine (both taken as needed), OTC testosterone supplements, OTC apple cider vinegar gummies BID and OTC ashwagandha gummies BID. Workup was negative for autoimmune, infectious, metabolic, obstructive and vascular causes of liver injury. Acetaminophen level and urine drug screen were negative. Trans-jugular liver biopsy revealed evidence of acute portal and lobular hepatitis and cholestasis, suggestive of DILI. Patient's liver enzymes continued to downtrend (AST=929, ALT=765, Tbili=22.5) after withdrawal of his supplements and was discharged.
Discussion: Ashwagandha causing DILI has been infrequently reported in the
literature. One case series of 5 patients in Iceland and a single case in Japan reported
that ashwagandha is an uncommon culprit linked to acute liver injury. None of the
reported cases necessitated liver transplant. Average time to resolution and
normalization of LFTs was 3.5 months and R values usually ranged in the mixed
range (R value 2-5). The case series also revealed that ashwagandha's toxicity could
be dose dependent, as higher peaks in LFT's were tied to recent increases in
ashwagandha dosing. Although ashwagandha has been a rarely reported cause of
DILI, a thorough history of medication and OTC supplements in patients with
undifferentiated acute liver injury may yield more cases. Further study and reporting
of DILI in the ever-growing supplement market appears to be required to minimize
potential injury.

Figure: Tranjugular Liver Biopsy Findings
Disclosures:
James Gnecco indicated no relevant financial relationships.
Hasan Baher indicated no relevant financial relationships.
Brenda Briones indicated no relevant financial relationships.
Fred Poordad indicated no relevant financial relationships.
James Gnecco, DO1, Hasan Baher, MD1, Brenda M. Briones, MD1, Fred Poordad, MD2. C0587 - Ashwagandha Toxicity: A Rare Case of Drug Induced Liver Injury (DILI), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
