Back


Poster Session D - Tuesday Morning
Category: Colon
D0094 - Recurrent Clostridioides difficile Infection in Patients Treated With Bezlotoxumab: Systematic Review and Meta-Analysis
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Mouhand F. Mohamed, MD, MSc
Warren Alpert Medical School of Brown University
Providence, RI
Presenting Author(s)
Mouhand F. Mohamed, MD, MSc1, Christopher Ward, MBBS2, Azizullah Beran, MD3, Mohamed Abdallah, MD4, Colleen Kelly, MD, FACG1
1Warren Alpert Medical School of Brown University, Providence, RI; 2Warren Alpert Medical School of Brown University, East Providence, RI; 3University of Toledo, Toledo, OH; 4University of Minnesota Medical Center, Minneapolis, MN
Introduction: Clostridioides difficile infection (CDI) remains a global health concern. Bezlotoxumab (BEZ) is a monoclonal antibody against C. difficile toxin B. Two randomized controlled trials (RCTs), MODIFY I & II, confirmed BEZ efficacy in preventing recurrent CDI (rCDI). Observational studies have since been conducted, and it is essential to explore the consistency of BEZ effectiveness utilizing these real-world data.
Methods: We performed a systematic review and meta-analysis aiming to pool the frequency of rCDI in patients receiving BEZ and explore BEZ efficacy in preventing rCDI compared to standard of care (SOC). We searched PubMed, EMBASE, Cochrane Library, and Google Scholar from inception through January 2022 for relevant RCTs or observational studies assessing BEZ in preventing rCDI. Single-arm studies describing experience with BEZ in preventing rCDI were also included for proportion-meta-analysis. A proportion meta-analysis with a random-effects model was used to pool rCDI prevalence with its corresponding 95% confidence interval (CI). In a meta-analysis of efficacy, we pooled Odds ratios (OR) to compare BEZ vs. SOC in preventing rCDI. Heterogeneity was assessed using the Higgins I2 statistic (I2 values >50% implied the presence of significant heterogeneity. MetaXl software was utilized for statistical analysis.
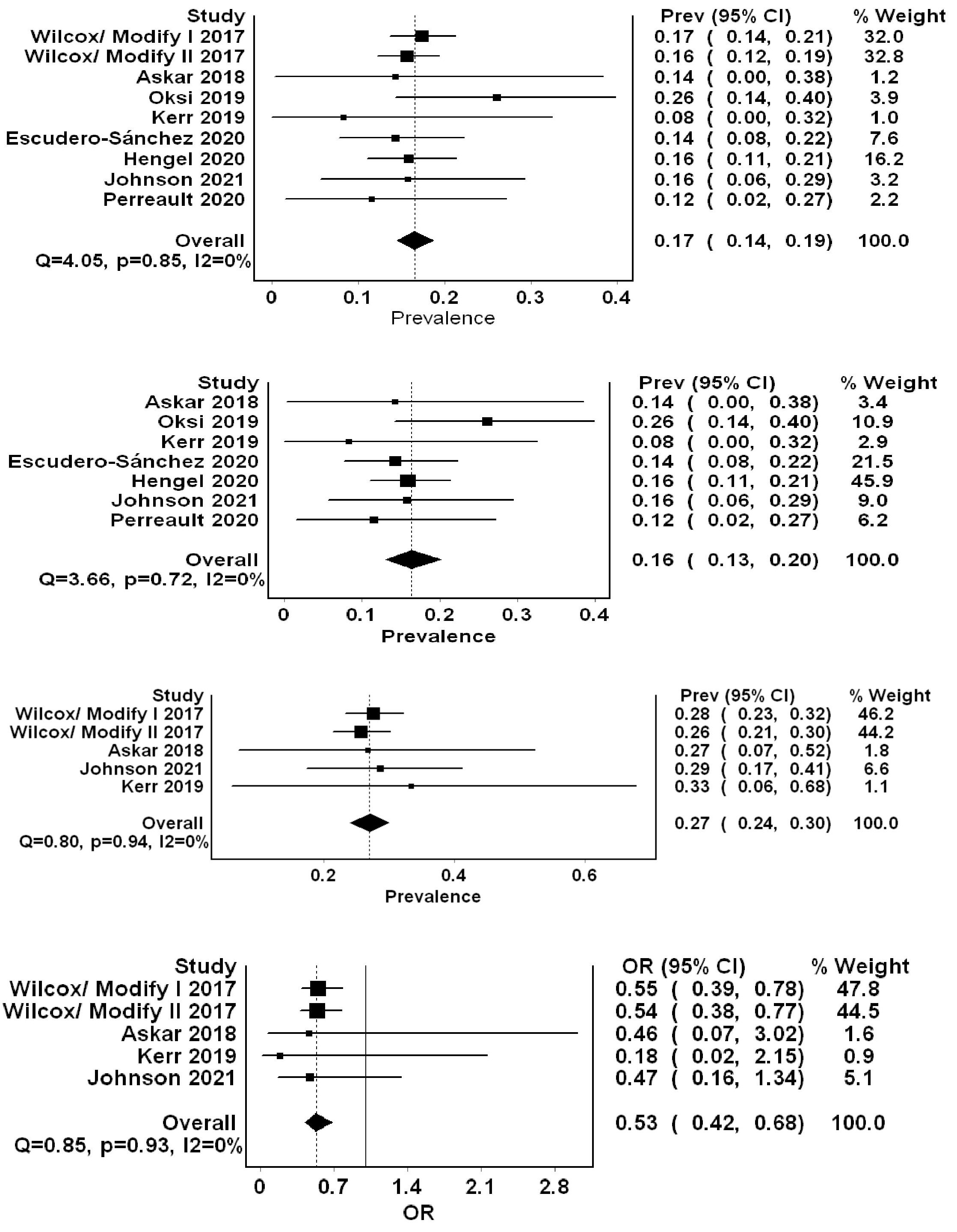
Results: Nine studies comprising two RCTs and 2056 patients, of which 1203 received BEZ, were included in the analysis. Of the constituent studies, five (1698 patients) compared BEZ vs. SOC. Pooled frequency of rCDI in patients receiving BEZ was 17% (95% CI = 0.14-0.19, I2=0%) (Figure 1). Subgroup analysis excluding RCTs resulted in 422 patients receiving BEZ with rCDI pooled frequency of 16% (95% CI = 0.13-0.20, I2=0%). We also pooled rCDI in the SOC group from studies that reported direct comparison with BEZ (5 studies=853 patients in the SOC group). Pooled rCDI rate was higher in the SOC group of 27% (17% (95% CI = 0.24-0.30, I20%). In the meta-analysis of efficacy, there was a significant reduction in rCDI in the BEZ treated group compared to SOC (OR= 0.53, 95% CI = 0.42-0.68, I20%). No heterogeneity was shown in all analyses, as depicted by an I2 of 0.
Discussion: Our meta-analysis comprising real-world data revealed lower rCDI in patients receiving BEZ and supported its efficacy in preventing rCDI compared to SOC. The results were consistent and homogenous. These results are keeping with the new ACG and IDSA guidelines that endorse a role for BEZ in the prevention of rCDI.
Disclosures:
Mouhand F. Mohamed, MD, MSc1, Christopher Ward, MBBS2, Azizullah Beran, MD3, Mohamed Abdallah, MD4, Colleen Kelly, MD, FACG1. D0094 - Recurrent Clostridioides difficile Infection in Patients Treated With Bezlotoxumab: Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Warren Alpert Medical School of Brown University, Providence, RI; 2Warren Alpert Medical School of Brown University, East Providence, RI; 3University of Toledo, Toledo, OH; 4University of Minnesota Medical Center, Minneapolis, MN
Introduction: Clostridioides difficile infection (CDI) remains a global health concern. Bezlotoxumab (BEZ) is a monoclonal antibody against C. difficile toxin B. Two randomized controlled trials (RCTs), MODIFY I & II, confirmed BEZ efficacy in preventing recurrent CDI (rCDI). Observational studies have since been conducted, and it is essential to explore the consistency of BEZ effectiveness utilizing these real-world data.
Methods: We performed a systematic review and meta-analysis aiming to pool the frequency of rCDI in patients receiving BEZ and explore BEZ efficacy in preventing rCDI compared to standard of care (SOC). We searched PubMed, EMBASE, Cochrane Library, and Google Scholar from inception through January 2022 for relevant RCTs or observational studies assessing BEZ in preventing rCDI. Single-arm studies describing experience with BEZ in preventing rCDI were also included for proportion-meta-analysis. A proportion meta-analysis with a random-effects model was used to pool rCDI prevalence with its corresponding 95% confidence interval (CI). In a meta-analysis of efficacy, we pooled Odds ratios (OR) to compare BEZ vs. SOC in preventing rCDI. Heterogeneity was assessed using the Higgins I2 statistic (I2 values >50% implied the presence of significant heterogeneity. MetaXl software was utilized for statistical analysis.
Results: Nine studies comprising two RCTs and 2056 patients, of which 1203 received BEZ, were included in the analysis. Of the constituent studies, five (1698 patients) compared BEZ vs. SOC. Pooled frequency of rCDI in patients receiving BEZ was 17% (95% CI = 0.14-0.19, I2=0%) (Figure 1). Subgroup analysis excluding RCTs resulted in 422 patients receiving BEZ with rCDI pooled frequency of 16% (95% CI = 0.13-0.20, I2=0%). We also pooled rCDI in the SOC group from studies that reported direct comparison with BEZ (5 studies=853 patients in the SOC group). Pooled rCDI rate was higher in the SOC group of 27% (17% (95% CI = 0.24-0.30, I20%). In the meta-analysis of efficacy, there was a significant reduction in rCDI in the BEZ treated group compared to SOC (OR= 0.53, 95% CI = 0.42-0.68, I20%). No heterogeneity was shown in all analyses, as depicted by an I2 of 0.
Discussion: Our meta-analysis comprising real-world data revealed lower rCDI in patients receiving BEZ and supported its efficacy in preventing rCDI compared to SOC. The results were consistent and homogenous. These results are keeping with the new ACG and IDSA guidelines that endorse a role for BEZ in the prevention of rCDI.

Figure: Forest plots summarizing the (A) overall pooled frequency of recurrent Clostridioides Difficile infections (rCDI) in patients treated with bezlotoxumab (Bez), (B) rCDI pooled frequency in patients treated with Bez utilizing real-world data only, (C) rCDI pooled frequency in standard of care (SOC) group, and (D) pooled odds ratio of rCDI in Bez treated patients compared to SOC.
Disclosures:
Mouhand Mohamed indicated no relevant financial relationships.
Christopher Ward indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Mohamed Abdallah indicated no relevant financial relationships.
Colleen Kelly: Finch Therapeutics – Grant/Research Support. OpenBiome – Clinical Advisory Board.
Mouhand F. Mohamed, MD, MSc1, Christopher Ward, MBBS2, Azizullah Beran, MD3, Mohamed Abdallah, MD4, Colleen Kelly, MD, FACG1. D0094 - Recurrent Clostridioides difficile Infection in Patients Treated With Bezlotoxumab: Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
