Back


Poster Session D - Tuesday Morning
Category: Colon
D0099 - Efficacy and Safety of RBX2660 in Reducing Recurrent Clostridioides difficile Infection in Patients With Underlying Gastrointestinal Comorbidities
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Sahil Khanna, MBBS, MS, FACG
Mayo Clinic
Rochester, MN
Presenting Author(s)
Sahil Khanna, MBBS, MS, FACG1, Erik R. Dubberke, MD, MSPH2, Whitfield L. Knapple, MD3, Paul Feuerstadt, MD, FACG4, Maha Assi, MD, MPH5, Samson Ng, PharmD6, Beth Guthmueller, AS7, Masakazu Ando, PhD6
1Mayo Clinic, Rochester, MN; 2Washington University School of Medicine, St. Louis, MO; 3Arkansas Gastroenterology, North Little Rock, AR; 4PACT Gastroenterology Center and Yale University School of Medicine, New Haven, CT; 5University of Kansas School of Medicine, Wichita, KS; 6Ferring Pharmaceuticals, Parsippany, NJ; 7Rebiotix Inc., a Ferring Company, Roseville, MN
Introduction: Several underlying gastrointestinal (GI) comorbidities are known risk factors for recurrent Clostridioides difficile infection (rCDI), yet patients with these GI comorbidities are often excluded from prospective clinical trials. Here, we report the efficacy and safety of RBX2660, a microbiota-based live biotherapeutic, in adult participants categorized by GI comorbidity subgroups (eg, past or present history of gastroesophageal reflux disease [GERD], irritable bowel syndrome [IBS], diverticulitis, inflammatory bowel disease [IBD], and unspecified colitis) from an interim analysis of PUNCH CD3-OLS, an ongoing open-label phase 3 study.
Methods: An ad hoc analysis of the modified intent-to-treat (mITT) population was conducted to identify subgroups according to GI comorbidities based on medical histories. Treatment success was defined as remaining free of CDI recurrence for 8 weeks after treatment. Participants were monitored for recurrence and treatment-emergent adverse events (TEAEs) for at least 6 months after treatment. Efficacy data are presented for the mITT population and safety data are presented for the safety population.
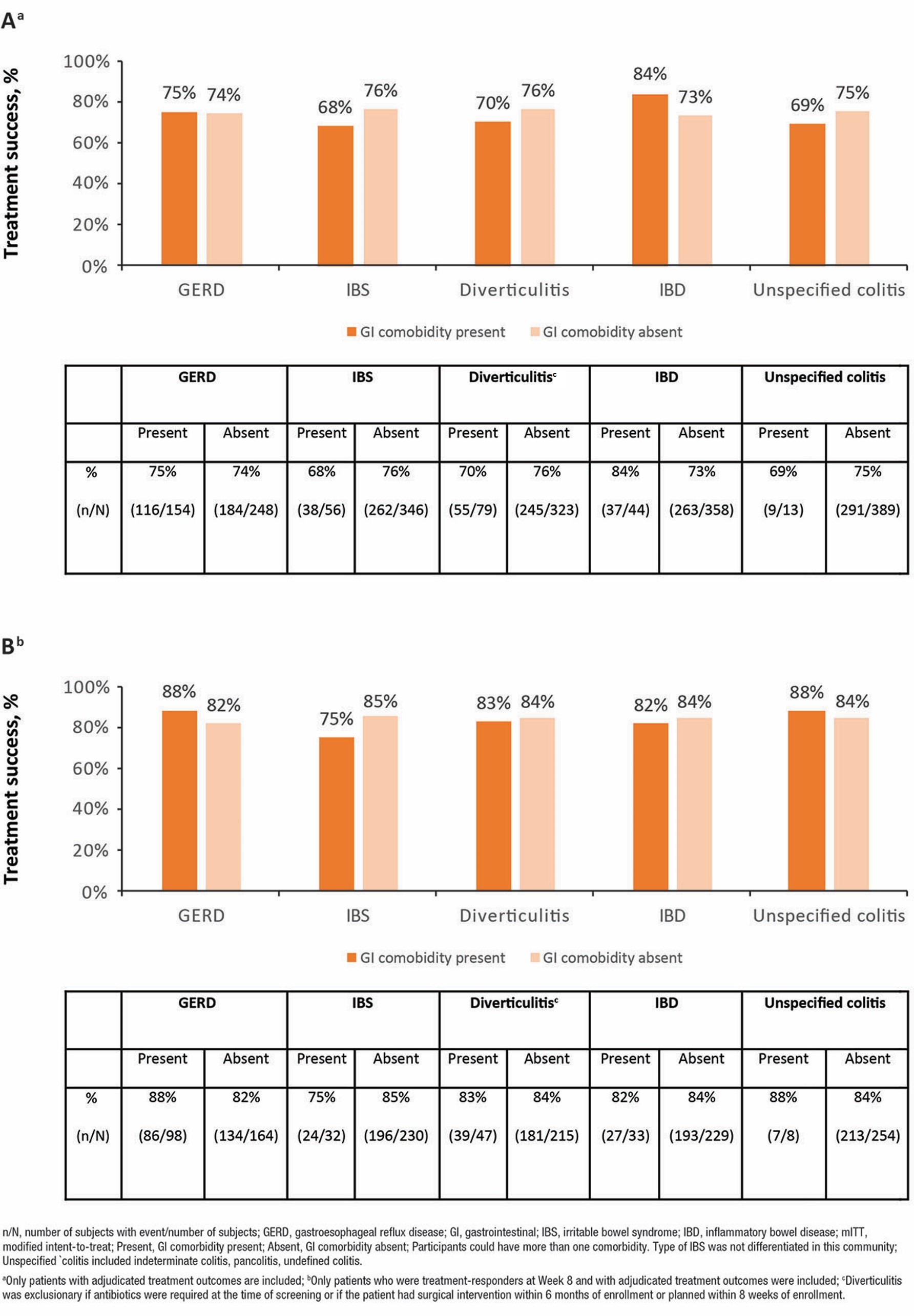
Results: At the time of this analysis, 402 of 469 screened participants in the mITT population were treated with RBX2660 and had adjudicated results, of which 300 of 402 (74.6%) had treatment success at Week 8. Across GI comorbidity subgroups, RBX2660 consistently reduced rCDI, and treatment success rates at Week 8 were comparable for participants with and without GI comorbidities (Fig. 1A). 6-month adjudicated results were available for 262 of 300 participants with treatment success at Week 8, of whom 220 (84.0%) remained CDI recurrence-free through 6 months. Sustained clinical response through 6 months was also maintained in RBX2660-responders with and without GI comorbidities (Fig. 1B). Overall AEs and TEAEs were similar between participants with and without GI comorbidities. TEAEs were experienced by 53.2% (100/188), 64.1% (41/64), 47.5% (47/99), 46.3% (25/54) and 52.9% (9/17) of participants with GERD, IBS, diverticulitis, IBD, and unspecified colitis, respectively. Most TEAEs were mild to moderate in severity and GI in nature (predominantly diarrhea and abdominal pain). Potentially life-threatening TEAEs and TEAEs leading to discontinuation were infrequently reported.
Discussion: These results suggest consistent efficacy and safety for RBX2660 in reducing rCDI in a patient population with underlying GI comorbidities.
Disclosures:
Sahil Khanna, MBBS, MS, FACG1, Erik R. Dubberke, MD, MSPH2, Whitfield L. Knapple, MD3, Paul Feuerstadt, MD, FACG4, Maha Assi, MD, MPH5, Samson Ng, PharmD6, Beth Guthmueller, AS7, Masakazu Ando, PhD6. D0099 - Efficacy and Safety of RBX2660 in Reducing Recurrent Clostridioides difficile Infection in Patients With Underlying Gastrointestinal Comorbidities, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Washington University School of Medicine, St. Louis, MO; 3Arkansas Gastroenterology, North Little Rock, AR; 4PACT Gastroenterology Center and Yale University School of Medicine, New Haven, CT; 5University of Kansas School of Medicine, Wichita, KS; 6Ferring Pharmaceuticals, Parsippany, NJ; 7Rebiotix Inc., a Ferring Company, Roseville, MN
Introduction: Several underlying gastrointestinal (GI) comorbidities are known risk factors for recurrent Clostridioides difficile infection (rCDI), yet patients with these GI comorbidities are often excluded from prospective clinical trials. Here, we report the efficacy and safety of RBX2660, a microbiota-based live biotherapeutic, in adult participants categorized by GI comorbidity subgroups (eg, past or present history of gastroesophageal reflux disease [GERD], irritable bowel syndrome [IBS], diverticulitis, inflammatory bowel disease [IBD], and unspecified colitis) from an interim analysis of PUNCH CD3-OLS, an ongoing open-label phase 3 study.
Methods: An ad hoc analysis of the modified intent-to-treat (mITT) population was conducted to identify subgroups according to GI comorbidities based on medical histories. Treatment success was defined as remaining free of CDI recurrence for 8 weeks after treatment. Participants were monitored for recurrence and treatment-emergent adverse events (TEAEs) for at least 6 months after treatment. Efficacy data are presented for the mITT population and safety data are presented for the safety population.
Results: At the time of this analysis, 402 of 469 screened participants in the mITT population were treated with RBX2660 and had adjudicated results, of which 300 of 402 (74.6%) had treatment success at Week 8. Across GI comorbidity subgroups, RBX2660 consistently reduced rCDI, and treatment success rates at Week 8 were comparable for participants with and without GI comorbidities (Fig. 1A). 6-month adjudicated results were available for 262 of 300 participants with treatment success at Week 8, of whom 220 (84.0%) remained CDI recurrence-free through 6 months. Sustained clinical response through 6 months was also maintained in RBX2660-responders with and without GI comorbidities (Fig. 1B). Overall AEs and TEAEs were similar between participants with and without GI comorbidities. TEAEs were experienced by 53.2% (100/188), 64.1% (41/64), 47.5% (47/99), 46.3% (25/54) and 52.9% (9/17) of participants with GERD, IBS, diverticulitis, IBD, and unspecified colitis, respectively. Most TEAEs were mild to moderate in severity and GI in nature (predominantly diarrhea and abdominal pain). Potentially life-threatening TEAEs and TEAEs leading to discontinuation were infrequently reported.
Discussion: These results suggest consistent efficacy and safety for RBX2660 in reducing rCDI in a patient population with underlying GI comorbidities.

Figure: Figure 1. (A) Treatment success at Week 8 in patients with and without GI comorbidities (mITT population; N=402); (B) Sustained treatment response through 6 months (mITT population; N=262).
Disclosures:
Sahil Khanna: Ferring Pharmaceuticals – Grant/Research Support. Finch – Grant/Research Support. Niche – Consultant. Pfizer – Grant/Research Support. Probiotech – Consultant. Seres Therapeutics – Grant/Research Support. Takeda/Shire – Consultant. Vedanta – Grant/Research Support.
Erik R. Dubberke: Abbott – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Rebiotix – Advisory Committee/Board Member. Seres – Consultant. Summit – Consultant. Synthetic Biologics – Grant/Research Support.
Whitfield L. Knapple indicated no relevant financial relationships.
Paul Feuerstadt: Ferring/Rebiotix Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck and Co – Consultant. Seres Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Maha Assi indicated no relevant financial relationships.
Samson Ng: Ferring Pharmaceuticals – Employee.
Beth Guthmueller: Rebiotix, a Ferring Company – Employee.
Masakazu Ando: Ferring Pharmaceuticals – Employee.
Sahil Khanna, MBBS, MS, FACG1, Erik R. Dubberke, MD, MSPH2, Whitfield L. Knapple, MD3, Paul Feuerstadt, MD, FACG4, Maha Assi, MD, MPH5, Samson Ng, PharmD6, Beth Guthmueller, AS7, Masakazu Ando, PhD6. D0099 - Efficacy and Safety of RBX2660 in Reducing Recurrent Clostridioides difficile Infection in Patients With Underlying Gastrointestinal Comorbidities, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
