Back

Poster Session D - Tuesday Morning
Category: Functional Bowel Disease
D0266 - Rifaximin Improves Both Fecal Urgency and Stool Consistency in Adults With Irritable Bowel Syndrome With Diarrhea (IBS-D): A Composite Endpoint Analysis of Two Randomized, Phase 3 Trials
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Brooks D. Cash, MD, FACG
University of Texas Health Science Center
Houston, TX
Presenting Author(s)
Brooks D. Cash, MD, FACG1, Kyle Staller, MD, MPH2, Leila Neshatian, MD, MSc3, Christopher Allen, MS4, Zeev Heimanson, PharmD4, Ali Rezaie, MD, MSc5
1University of Texas Health Science Center, Houston, TX; 2Massachusetts General Hospital, Boston, MA; 3Stanford University School of Medicine, Stanford, CA; 4Salix Pharmaceuticals, Bridgewater, NJ; 5Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: For patients with IBS-D, in addition to loose stool consistency, fecal urgency is a common, bothersome symptom. The aim was to evaluate rifaximin for simultaneously improving symptoms of fecal urgency in addition to loose/watery stool consistency as a unique composite bowel symptom endpoint.
Methods: A post hoc analysis was conducted with data from 2 identically designed, phase 3, randomized, double-blind trials. Adults with IBS-D, with screening daily mean stool consistency score of ≥3.5 (range, 1 “very hard”; 5 “watery”), took rifaximin 550 mg TID or placebo for 2 weeks, followed by a 4-week treatment-free phase to assess response. Fecal urgency was based on patient response to the daily question, “Have you felt or experienced a sense of urgency today?” Composite bowel symptom responders were defined as patients who simultaneously achieved a ≥30% decrease from baseline in the percentage of days with fecal urgency and had a mean weekly stool consistency score of < 4 for ≥2 of the first 4 post-treatment weeks. Data were analyzed using last observation carried forward; P values were calculated using the Cochran-Mantel-Haenszel method, adjusting for analysis center.
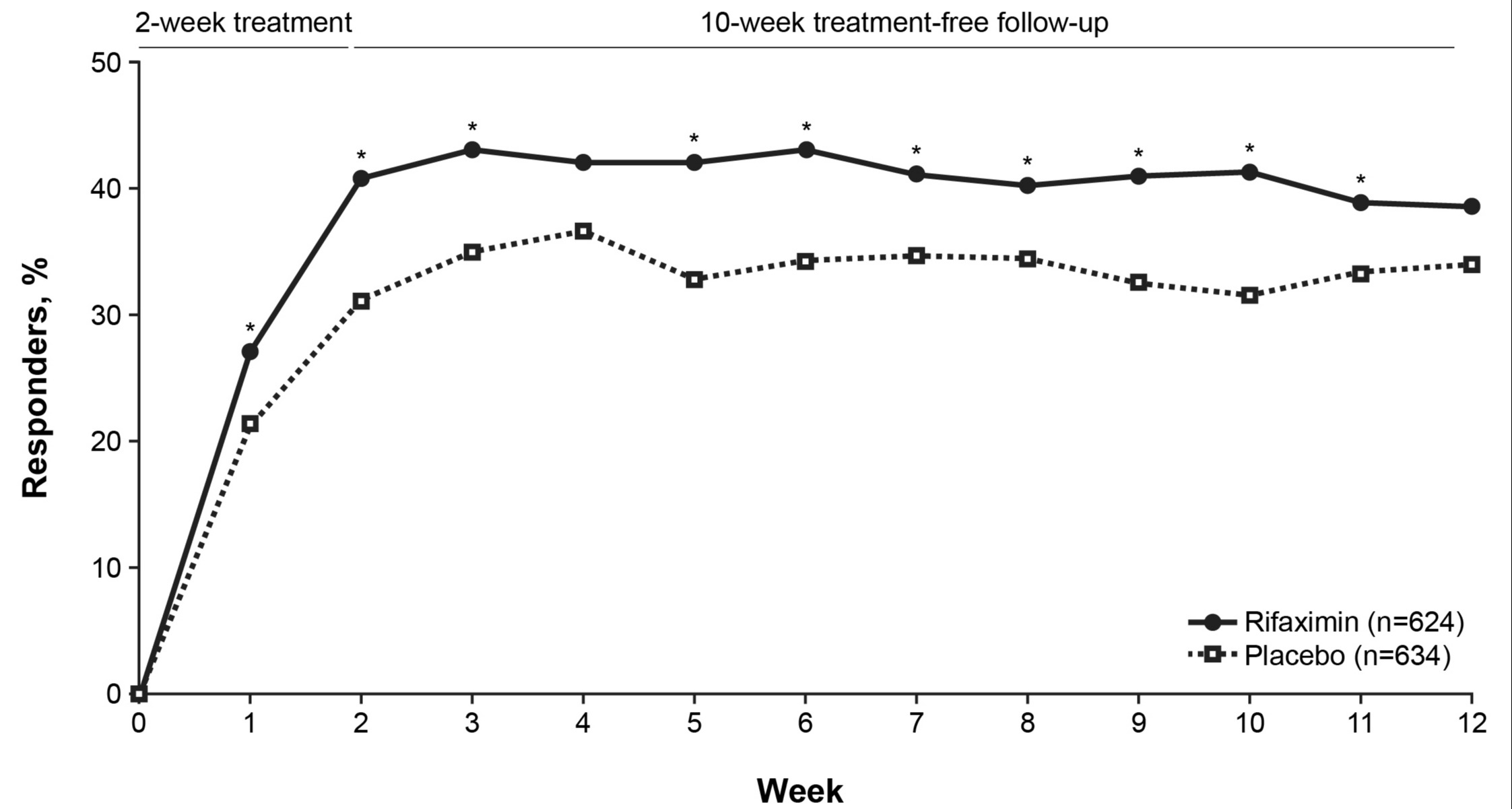
Results: A total of 1258 adults (rifaximin [n=624], placebo [n=634]) were included (mean ± SD age, 45.9 ± 14.5 years; 72.3% female). Similar values at baseline were observed for rifaximin and placebo groups for the percentage of days with fecal urgency (82%), mean daily stool consistency score (3.9), and mean ± SD number of daily bowel movements (3.0 ± 1.5). A significantly greater percentage of patients treated with rifaximin were bowel symptom composite responders vs placebo for ≥2 of the first 4 weeks post-treatment (47.9% vs 39.3%, respectively; P=0.002). A higher percentage of responders with rifaximin was observed vs placebo when analyzed by week (Figure). A significantly higher percentage of patients who were bowel symptom composite responders during ≥2 of the first 4 weeks post-treatment maintained response during ≥3 of the additional 6 weeks of treatment-free follow up (Week 12) in rifaximin vs placebo groups (44.7% vs 36.1%; P=0.002). For the individual components of response, a significantly greater percentage of patients in rifaximin group were fecal urgency responders vs placebo (52.9% vs 43.1%; P< 0.001) or stool consistency responders (82.7% vs 77.8%; P=0.03).
Discussion: A 2-week course of rifaximin significantly and simultaneously improved fecal urgency and stool consistency vs placebo in adults with IBS-D.
Disclosures:
Brooks D. Cash, MD, FACG1, Kyle Staller, MD, MPH2, Leila Neshatian, MD, MSc3, Christopher Allen, MS4, Zeev Heimanson, PharmD4, Ali Rezaie, MD, MSc5. D0266 - Rifaximin Improves Both Fecal Urgency and Stool Consistency in Adults With Irritable Bowel Syndrome With Diarrhea (IBS-D): A Composite Endpoint Analysis of Two Randomized, Phase 3 Trials, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Texas Health Science Center, Houston, TX; 2Massachusetts General Hospital, Boston, MA; 3Stanford University School of Medicine, Stanford, CA; 4Salix Pharmaceuticals, Bridgewater, NJ; 5Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: For patients with IBS-D, in addition to loose stool consistency, fecal urgency is a common, bothersome symptom. The aim was to evaluate rifaximin for simultaneously improving symptoms of fecal urgency in addition to loose/watery stool consistency as a unique composite bowel symptom endpoint.
Methods: A post hoc analysis was conducted with data from 2 identically designed, phase 3, randomized, double-blind trials. Adults with IBS-D, with screening daily mean stool consistency score of ≥3.5 (range, 1 “very hard”; 5 “watery”), took rifaximin 550 mg TID or placebo for 2 weeks, followed by a 4-week treatment-free phase to assess response. Fecal urgency was based on patient response to the daily question, “Have you felt or experienced a sense of urgency today?” Composite bowel symptom responders were defined as patients who simultaneously achieved a ≥30% decrease from baseline in the percentage of days with fecal urgency and had a mean weekly stool consistency score of < 4 for ≥2 of the first 4 post-treatment weeks. Data were analyzed using last observation carried forward; P values were calculated using the Cochran-Mantel-Haenszel method, adjusting for analysis center.
Results: A total of 1258 adults (rifaximin [n=624], placebo [n=634]) were included (mean ± SD age, 45.9 ± 14.5 years; 72.3% female). Similar values at baseline were observed for rifaximin and placebo groups for the percentage of days with fecal urgency (82%), mean daily stool consistency score (3.9), and mean ± SD number of daily bowel movements (3.0 ± 1.5). A significantly greater percentage of patients treated with rifaximin were bowel symptom composite responders vs placebo for ≥2 of the first 4 weeks post-treatment (47.9% vs 39.3%, respectively; P=0.002). A higher percentage of responders with rifaximin was observed vs placebo when analyzed by week (Figure). A significantly higher percentage of patients who were bowel symptom composite responders during ≥2 of the first 4 weeks post-treatment maintained response during ≥3 of the additional 6 weeks of treatment-free follow up (Week 12) in rifaximin vs placebo groups (44.7% vs 36.1%; P=0.002). For the individual components of response, a significantly greater percentage of patients in rifaximin group were fecal urgency responders vs placebo (52.9% vs 43.1%; P< 0.001) or stool consistency responders (82.7% vs 77.8%; P=0.03).
Discussion: A 2-week course of rifaximin significantly and simultaneously improved fecal urgency and stool consistency vs placebo in adults with IBS-D.

Figure: Figure. Percentage of patients with ≥30% decrease from baseline in percentage of days with fecal urgency and mean weekly stool consistency score of < 4, by week
*P< 0.05 vs placebo.
*P< 0.05 vs placebo.
Disclosures:
Brooks Cash: AbbVie – Consultant, Speakers Bureau. Ardelyx – Consultant, Speakers Bureau. RedHill – Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Kyle Staller: Arena Pharmaceuticals, Gelesis, GI Supply, and Shire (Takeda Pharmaceutical Company Ltd) – Consultant. Ironwood Pharmaceuticals, Inc. and Urovant Sciences, Inc. – Grant/Research Support.
Leila Neshatian indicated no relevant financial relationships.
Christopher Allen: Salix Pharmaceuticals – Employee.
Zeev Heimanson: Salix Pharmaceuticals – Employee.
Ali Rezaie: Gemelli Biotech – Equity. Salix Pharmaceuticals – Consultant, Grant/Research Support.
Brooks D. Cash, MD, FACG1, Kyle Staller, MD, MPH2, Leila Neshatian, MD, MSc3, Christopher Allen, MS4, Zeev Heimanson, PharmD4, Ali Rezaie, MD, MSc5. D0266 - Rifaximin Improves Both Fecal Urgency and Stool Consistency in Adults With Irritable Bowel Syndrome With Diarrhea (IBS-D): A Composite Endpoint Analysis of Two Randomized, Phase 3 Trials, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
