Back

Poster Session D - Tuesday Morning
Category: IBD
D0421 - A Rare Etiology for Ulcerative Colitis Flare
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Patrick J. Tempera, DO
Albany Medical College
Albany, NY
Presenting Author(s)
Patrick J. Tempera, DO, Maheep Sangha, MBBS, Asra Batool, MBBS
Albany Medical Center, Albany, NY
Introduction: Prior to colonoscopy, it is well understood that patients must undergo bowel cleansing. Based on the type of laxative, colonoscopy preparations fall into two categories – polymer-based formulas (PEG) and saline-based formulas (NaP). Both types of bowel preparations are deemed to be relatively safe and part of routine practice. However, we describe the rare case of an ulcerative colitis (UC) flare due to the bowel preparation formula.
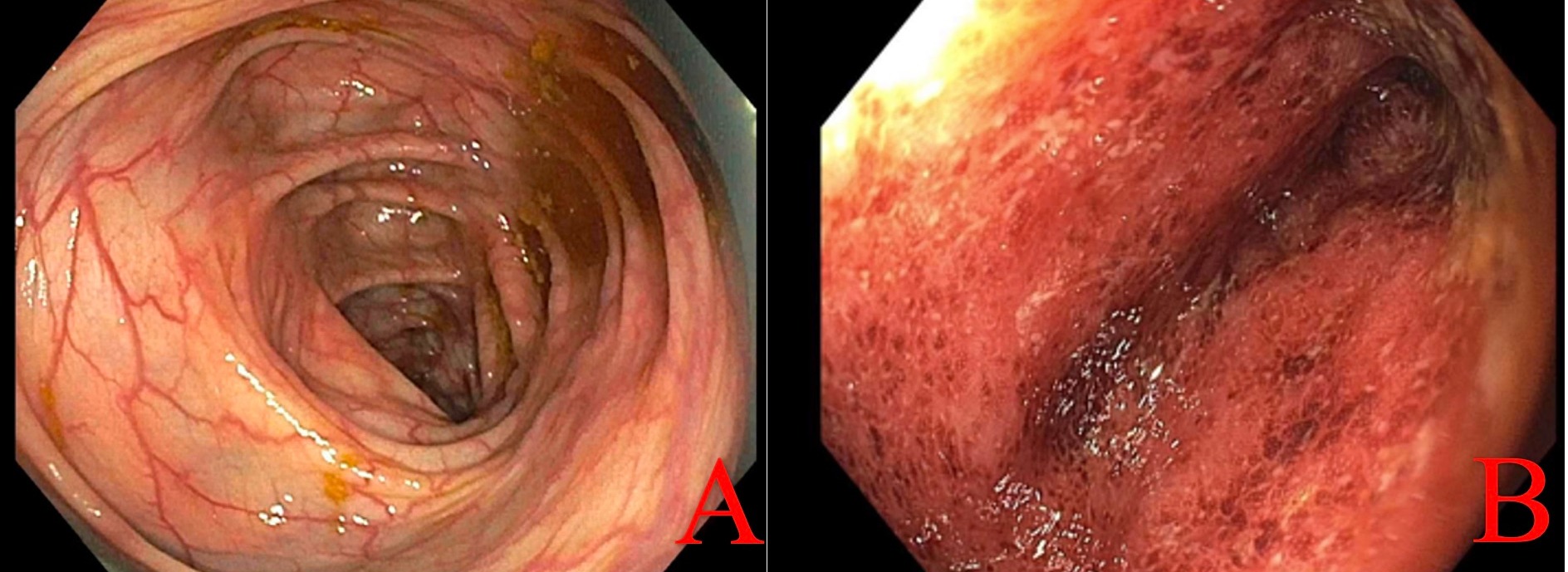
Case Description/Methods: A 29-year-old female with diagnosis of UC, presently in clinical and biochemical remission on oral mesalamine, contracted COVID-19 and had reactivation of UC symptoms. After being on budesonide tablets and rectal foam for two months, patient achieved clinical remission, and a surveillance colonoscopy was performed which revealed normal colon and terminal ileum except mild congestion in the cecum (Figure A). Pathology revealed unremarkable mucosa in the entire colon except for chronic active colitis in the cecum. Immediately following this colonoscopy, the patient started to experience another severe UC flare requiring hospitalization. The patient’s laboratory work-up was normal except for an elevated fecal calprotectin (1710). Stool infectious work-up was negative and the patient denied any NSAID or antibiotic use. The patient underwent a repeat colonoscopy which revealed severe Mayo 3 pancolitis (Figure B) in comparison to a stable colonoscopy a few weeks prior. It was revealed that for her initial colonoscopy, she had used SUPREP bowel prep kit. On prior colonoscopies she had used MiraLAX bowel prep with no adverse effects. During hospitalization, the patient was started on biologic therapy with good effect.
Discussion: There are no clear guidelines on appropriate bowel preparation formula for the inflammatory bowel disease (IBD) population. Sufficient literature exists to confirm that NaP can irritate the intestinal mucosal wall. Moreover, numerous animal experiments have employed dextran sodium sulfate for chemical induction of intestinal inflammation to mimic UC flares in humans [1]. Thus, it can be surmised that because SUPREP ingredients contain sodium sulfate, the potential for UC flare is higher. It is pertinent for practitioners to be aware of the possible rare adverse effects of saline-based formulas, especially when treating the IBD population.
[1] Eichele DD, et al., Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of IBD pathogenesis, 2017, 6016-6029.
Disclosures:
Patrick J. Tempera, DO, Maheep Sangha, MBBS, Asra Batool, MBBS. D0421 - A Rare Etiology for Ulcerative Colitis Flare, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Albany Medical Center, Albany, NY
Introduction: Prior to colonoscopy, it is well understood that patients must undergo bowel cleansing. Based on the type of laxative, colonoscopy preparations fall into two categories – polymer-based formulas (PEG) and saline-based formulas (NaP). Both types of bowel preparations are deemed to be relatively safe and part of routine practice. However, we describe the rare case of an ulcerative colitis (UC) flare due to the bowel preparation formula.
Case Description/Methods: A 29-year-old female with diagnosis of UC, presently in clinical and biochemical remission on oral mesalamine, contracted COVID-19 and had reactivation of UC symptoms. After being on budesonide tablets and rectal foam for two months, patient achieved clinical remission, and a surveillance colonoscopy was performed which revealed normal colon and terminal ileum except mild congestion in the cecum (Figure A). Pathology revealed unremarkable mucosa in the entire colon except for chronic active colitis in the cecum. Immediately following this colonoscopy, the patient started to experience another severe UC flare requiring hospitalization. The patient’s laboratory work-up was normal except for an elevated fecal calprotectin (1710). Stool infectious work-up was negative and the patient denied any NSAID or antibiotic use. The patient underwent a repeat colonoscopy which revealed severe Mayo 3 pancolitis (Figure B) in comparison to a stable colonoscopy a few weeks prior. It was revealed that for her initial colonoscopy, she had used SUPREP bowel prep kit. On prior colonoscopies she had used MiraLAX bowel prep with no adverse effects. During hospitalization, the patient was started on biologic therapy with good effect.
Discussion: There are no clear guidelines on appropriate bowel preparation formula for the inflammatory bowel disease (IBD) population. Sufficient literature exists to confirm that NaP can irritate the intestinal mucosal wall. Moreover, numerous animal experiments have employed dextran sodium sulfate for chemical induction of intestinal inflammation to mimic UC flares in humans [1]. Thus, it can be surmised that because SUPREP ingredients contain sodium sulfate, the potential for UC flare is higher. It is pertinent for practitioners to be aware of the possible rare adverse effects of saline-based formulas, especially when treating the IBD population.
[1] Eichele DD, et al., Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of IBD pathogenesis, 2017, 6016-6029.

Figure: Figure A: Colon (04/19/2022) and Figure B: Colon (05/09/2022).
Disclosures:
Patrick Tempera indicated no relevant financial relationships.
Maheep Sangha indicated no relevant financial relationships.
Asra Batool indicated no relevant financial relationships.
Patrick J. Tempera, DO, Maheep Sangha, MBBS, Asra Batool, MBBS. D0421 - A Rare Etiology for Ulcerative Colitis Flare, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
