Back

Poster Session D - Tuesday Morning
Category: Liver
D0541 - New Drugs and New Toxicities: Liraglutide-Induced Liver Injury
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Rizwan Ishtiaq, MD
St. Francis Hospital and Medical Center
Hartford, CT
Presenting Author(s)
Faisal Inayat, MBBS1, Faisal Ibrahim, MBBS2, Muhammad Hassan Naeem Goraya, MBBS3, Rizwan Ishtiaq, MD4, Arslan Afzal, MD5, Gul Nawaz, MD6
1Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 2Wexham Park Hospital, Slough, England, United Kingdom; 3Allama Iqbal Medical College, Lahoe, Punjab, Pakistan; 4St. Francis Hospital and Medical Center, Hartford, CT; 5Woodhull Medical Center, Brooklyn, NY; 6Marshfield Medical Center, Marshfield, WI
Introduction: Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. It has demonstrated efficacy as an antidiabetic and anti-obesity drug. However, several common adverse events have also been reported, including nausea, vomiting, loose stools, and rarely pancreatitis. To our knowledge, this report represents only the third case of drug-induced liver injury (DILI) after the use of liraglutide.
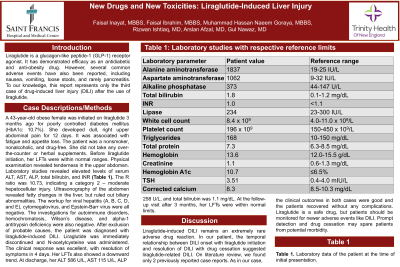
Case Description/Methods: A 43-year-old obese female was initiated on liraglutide 3 months ago for poorly controlled diabetes mellitus (HbA1c: 10.7%). She developed dull, right upper abdominal pain for 12 days. It was associated with fatigue and appetite loss. The patient was a nonsmoker, nonalcoholic, and drug-free. She did not take any over-the-counter or herbal supplements. Before liraglutide initiation, her LFTs were within normal ranges. Physical examination revealed tenderness in the upper abdomen. Laboratory studies revealed ALT 1837 U/L, AST 1062 U/L, ALP 373 U/L, total bilirubin 1.8 mg/ dL, and INR 1.0. The R ratio was 10.73, indicating a category 2 – moderate hepatocellular injury. Ultrasonography of the abdomen revealed fatty changes in the liver, but ruled out biliary abnormalities. The workup for infectious hepatitis (A, B, C, D, and E), cytomegalovirus, and Epstein-Barr virus were all negative. The investigations for autoimmune disorders, hemochromatosis, Wilson’s disease, and alpha-1 antitrypsin deficiency were also negative. After exclusion of probable causes, the patient was diagnosed with liraglutide-induced DILI. Liraglutide was immediately discontinued and N-acetylcysteine was administered. The clinical response was excellent, with resolution of symptoms in 4 days. Her LFTs also showed a downward trend. At discharge, her ALT 586 U/L, AST 115 U/L, ALP 258 U/L, and total bilirubin was 1.1 mg/dL. At the follow-up visit after 3 months, her LFTs were within normal limits.
Discussion: Liraglutide-induced DILI remains an extremely rare adverse drug reaction. In our patient, the temporal relationship between DILI onset with liraglutide initiation and resolution of DILI with drug cessation indicated liraglutide-related DILI. On literature review, we found only 2 previously reported case reports. As in our case, the clinical outcomes in both cases were good and the patients recovered without any complications. Liraglutide is a safe drug, but patients should be monitored for newer adverse events like DILI. Prompt detection and drug cessation may spare patients from potential morbidity.
Disclosures:
Faisal Inayat, MBBS1, Faisal Ibrahim, MBBS2, Muhammad Hassan Naeem Goraya, MBBS3, Rizwan Ishtiaq, MD4, Arslan Afzal, MD5, Gul Nawaz, MD6. D0541 - New Drugs and New Toxicities: Liraglutide-Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 2Wexham Park Hospital, Slough, England, United Kingdom; 3Allama Iqbal Medical College, Lahoe, Punjab, Pakistan; 4St. Francis Hospital and Medical Center, Hartford, CT; 5Woodhull Medical Center, Brooklyn, NY; 6Marshfield Medical Center, Marshfield, WI
Introduction: Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. It has demonstrated efficacy as an antidiabetic and anti-obesity drug. However, several common adverse events have also been reported, including nausea, vomiting, loose stools, and rarely pancreatitis. To our knowledge, this report represents only the third case of drug-induced liver injury (DILI) after the use of liraglutide.
Case Description/Methods: A 43-year-old obese female was initiated on liraglutide 3 months ago for poorly controlled diabetes mellitus (HbA1c: 10.7%). She developed dull, right upper abdominal pain for 12 days. It was associated with fatigue and appetite loss. The patient was a nonsmoker, nonalcoholic, and drug-free. She did not take any over-the-counter or herbal supplements. Before liraglutide initiation, her LFTs were within normal ranges. Physical examination revealed tenderness in the upper abdomen. Laboratory studies revealed ALT 1837 U/L, AST 1062 U/L, ALP 373 U/L, total bilirubin 1.8 mg/ dL, and INR 1.0. The R ratio was 10.73, indicating a category 2 – moderate hepatocellular injury. Ultrasonography of the abdomen revealed fatty changes in the liver, but ruled out biliary abnormalities. The workup for infectious hepatitis (A, B, C, D, and E), cytomegalovirus, and Epstein-Barr virus were all negative. The investigations for autoimmune disorders, hemochromatosis, Wilson’s disease, and alpha-1 antitrypsin deficiency were also negative. After exclusion of probable causes, the patient was diagnosed with liraglutide-induced DILI. Liraglutide was immediately discontinued and N-acetylcysteine was administered. The clinical response was excellent, with resolution of symptoms in 4 days. Her LFTs also showed a downward trend. At discharge, her ALT 586 U/L, AST 115 U/L, ALP 258 U/L, and total bilirubin was 1.1 mg/dL. At the follow-up visit after 3 months, her LFTs were within normal limits.
Discussion: Liraglutide-induced DILI remains an extremely rare adverse drug reaction. In our patient, the temporal relationship between DILI onset with liraglutide initiation and resolution of DILI with drug cessation indicated liraglutide-related DILI. On literature review, we found only 2 previously reported case reports. As in our case, the clinical outcomes in both cases were good and the patients recovered without any complications. Liraglutide is a safe drug, but patients should be monitored for newer adverse events like DILI. Prompt detection and drug cessation may spare patients from potential morbidity.
Disclosures:
Faisal Inayat indicated no relevant financial relationships.
Faisal Ibrahim indicated no relevant financial relationships.
Muhammad Hassan Naeem Goraya indicated no relevant financial relationships.
Rizwan Ishtiaq indicated no relevant financial relationships.
Arslan Afzal indicated no relevant financial relationships.
Gul Nawaz indicated no relevant financial relationships.
Faisal Inayat, MBBS1, Faisal Ibrahim, MBBS2, Muhammad Hassan Naeem Goraya, MBBS3, Rizwan Ishtiaq, MD4, Arslan Afzal, MD5, Gul Nawaz, MD6. D0541 - New Drugs and New Toxicities: Liraglutide-Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
