Back

Poster Session B - Monday Morning
Category: Small Intestine
B0680 - Ileocolitis Associated With the Checkpoint Inhibitor Palbociclib (PAL)
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Christina Gomez, MD
University of Missouri Kansas City School of Medicine
Kansas City, MO
Presenting Author(s)
Christina Gomez, MD1, Thomas Bierman, MD1, Wendell Clarkston, MD2
1University of Missouri Kansas City School of Medicine, Kansas City, MO; 2University of Missouri-Kansas City School of Medicine, Saint Luke's Hospital, Kansas City, MO
Introduction: Cancer treatments are continuously being updated to optimize survival. Newer checkpoint inhibitors (CDK4/6 inhibitors) have demonstrated promising responses in ER-positive breast cancers. We report a rare side effect of ileocolitis with Palbociclib (PAL) treatment.
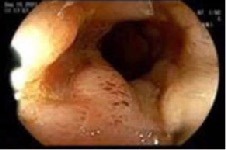
Case Description/Methods: A 30-year-old female with biopsy confirmed stage IV ER+, PR+, HER2- ductal breast carcinoma with metastasis to liver and bone presented with abdominal pain, diarrhea, and hematochezia 2 months into therapy with letrozole and PAL. Subsequent colonoscopy 4 months after onset of symptoms showed diffuse colitis with congestion, erythema, and shallow ulceration scattered through the whole colon, and several superficial aphthous ulcerations in the ileum. Stool for C. difficile by PCR was negative and biopsies revealed nonspecific ileitis and colitis. There was concern for IBD vs drug induced enteritis/colitis. PAL was held and the patient was started on prednisone taper with resolution of symptoms after 1 month. Given concern for disease progression, PAL was restarted 1 month later with return of symptoms and hospital admission. Laboratory studies showed WBC 1.84 TH/uL, Hgb 7.6 g/dL, plt 24 TH/uL, LDH 4211 U/L, ALT 450 U/L, AST 221 U/L, alk phos 223 U/L, total bilirubin 1.3 mg/dL, ESR 49 mm/hr and CRP 45 mg/L. Infection was excluded via stool studies including C. difficile PCR and blood culture. Colonoscopy was performed and showed erythema, friability, congestion, erosions, and ulceration in the TI compatible with ileitis (Image1) as well as patchy erythema, edema, erosions, and ulcers throughout the colon with skip lesions and sparing of the rectum. Biopsies were reported as normal TI and colon. PAL was discontinued indefinitely and the patient had complete resolution of symptoms within weeks.
Discussion: CDK4/6 inhibitors are new first-line treatment for metastatic HR-positive breast cancer. There are no prior reports of PAL induced enterocolitis without concomitant radiation. In our patient, symptoms could not be easily distinguished from infectious colitis or IBD clinically. Ileal and colonic biopsies showed nonspecific ileitis and colitis or were reported as normal. As CDK4/6 inhibitors become used more as the first-line treatment of metastatic HR-positive breast cancer, additional evidence of GI related toxicities may emerge. Checkpoint inhibitor induced ileitis/colitis should be considered in a patient with new onset hematochezia and diarrhea after initiation of these agents.
Disclosures:
Christina Gomez, MD1, Thomas Bierman, MD1, Wendell Clarkston, MD2. B0680 - Ileocolitis Associated With the Checkpoint Inhibitor Palbociclib (PAL), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Missouri Kansas City School of Medicine, Kansas City, MO; 2University of Missouri-Kansas City School of Medicine, Saint Luke's Hospital, Kansas City, MO
Introduction: Cancer treatments are continuously being updated to optimize survival. Newer checkpoint inhibitors (CDK4/6 inhibitors) have demonstrated promising responses in ER-positive breast cancers. We report a rare side effect of ileocolitis with Palbociclib (PAL) treatment.
Case Description/Methods: A 30-year-old female with biopsy confirmed stage IV ER+, PR+, HER2- ductal breast carcinoma with metastasis to liver and bone presented with abdominal pain, diarrhea, and hematochezia 2 months into therapy with letrozole and PAL. Subsequent colonoscopy 4 months after onset of symptoms showed diffuse colitis with congestion, erythema, and shallow ulceration scattered through the whole colon, and several superficial aphthous ulcerations in the ileum. Stool for C. difficile by PCR was negative and biopsies revealed nonspecific ileitis and colitis. There was concern for IBD vs drug induced enteritis/colitis. PAL was held and the patient was started on prednisone taper with resolution of symptoms after 1 month. Given concern for disease progression, PAL was restarted 1 month later with return of symptoms and hospital admission. Laboratory studies showed WBC 1.84 TH/uL, Hgb 7.6 g/dL, plt 24 TH/uL, LDH 4211 U/L, ALT 450 U/L, AST 221 U/L, alk phos 223 U/L, total bilirubin 1.3 mg/dL, ESR 49 mm/hr and CRP 45 mg/L. Infection was excluded via stool studies including C. difficile PCR and blood culture. Colonoscopy was performed and showed erythema, friability, congestion, erosions, and ulceration in the TI compatible with ileitis (Image1) as well as patchy erythema, edema, erosions, and ulcers throughout the colon with skip lesions and sparing of the rectum. Biopsies were reported as normal TI and colon. PAL was discontinued indefinitely and the patient had complete resolution of symptoms within weeks.
Discussion: CDK4/6 inhibitors are new first-line treatment for metastatic HR-positive breast cancer. There are no prior reports of PAL induced enterocolitis without concomitant radiation. In our patient, symptoms could not be easily distinguished from infectious colitis or IBD clinically. Ileal and colonic biopsies showed nonspecific ileitis and colitis or were reported as normal. As CDK4/6 inhibitors become used more as the first-line treatment of metastatic HR-positive breast cancer, additional evidence of GI related toxicities may emerge. Checkpoint inhibitor induced ileitis/colitis should be considered in a patient with new onset hematochezia and diarrhea after initiation of these agents.

Figure: Image 1: Erythema, friability, congestion, erosions, and ulceration in the terminal ileum
Disclosures:
Christina Gomez indicated no relevant financial relationships.
Thomas Bierman indicated no relevant financial relationships.
Wendell Clarkston indicated no relevant financial relationships.
Christina Gomez, MD1, Thomas Bierman, MD1, Wendell Clarkston, MD2. B0680 - Ileocolitis Associated With the Checkpoint Inhibitor Palbociclib (PAL), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
