Back

Poster Session B - Monday Morning
Category: Liver
B0576 - It Only Takes One: A Case of Drug-Induced Liver Injury Due to Cefazolin Administration
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Patrick Lazarczyk, DO
Ascension Providence Hospital
Southfield, Michigan
Presenting Author(s)
Patrick Lazarczyk, DO1, Eric M. Sieloff, MD1, Janice Fields, MD, FACG2
1Ascension Providence Hospital, Southfield, MI; 2Ascension Providence, Southfield, MI
Introduction: Drug-induced liver injury (DILI) is a common cause of hepatotoxicity that has been associated with multiple medications and supplements. Herein, we present an intriguing case of DILI related to preoperative Cefazolin administration.
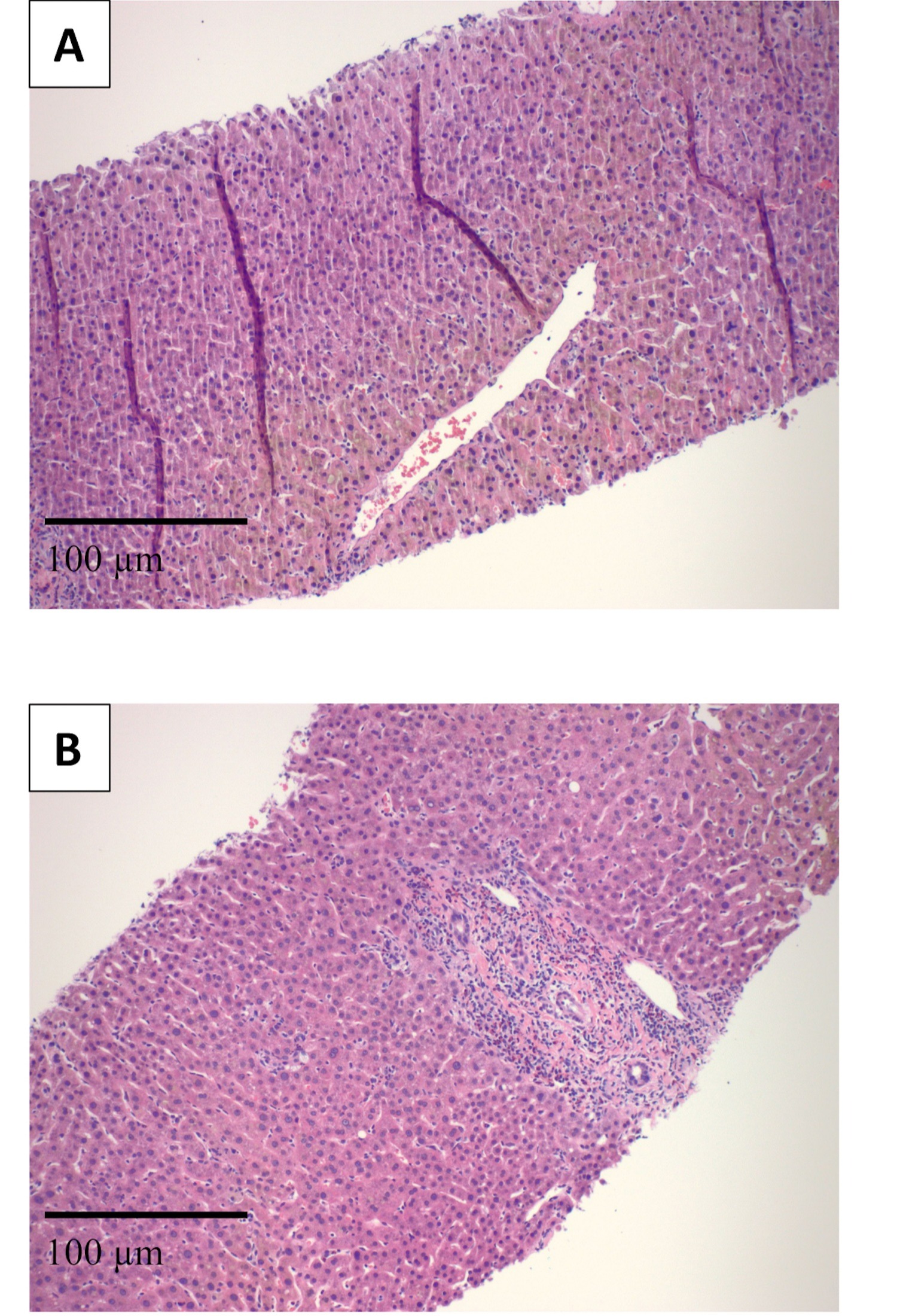
Case Description/Methods: A 64-year-old female with history of breast cancer presented for right upper quadrant abdominal pain, nausea, vomiting, and jaundice for 1 week. The patient underwent left mastectomy with administration of a single dose of preoperative IV Cefazolin about three weeks prior to presentation. On presentation, vital signs were within normal limits. On physical exam, the patient was noticeably jaundiced with right upper quadrant tenderness. Laboratory studies showed total bilirubin 11.9 mg/dL, direct bilirubin 8 mg/dL, alkaline phosphatase 603 IU/L, AST 165 IU/L, ALT 376 IU/L. Abdominal ultrasound found no biliary obstruction. Magnetic resonance cholangiopancreatography noted mild hepatomegaly with no intrahepatic or extrahepatic ductal dilatation. Workup for autoimmune disease, Wilson disease, and hemochromatosis was negative. Testing for hepatitis A and C was negative. The patient had positive HBcAb, however, HBsAg, HBsAb, and quantitative DNA PCR were negative. Liver biopsy showed cholestatic hepatitis, no significant fibrosis, and prominent eosinophils consistent with DILI. The patient was diagnosed with DILI secondary to Cefazolin administration. She was ultimately discharged home with improvement of symptoms and repeat lab work showing normalization of liver chemistries.
Discussion: Cefazolin is an antibiotic that is widely used for surgical prophylaxis, and it can be overlooked when working up acute liver injury, as it is usually a one-time dose. According to LiverTox, the latency period between administration and onset of liver injury is typically 1-4 weeks with most cases causing mild elevations of liver enzymes, however, significant elevations in aminotransferases greater than 5 times the upper limit of normal are rare (< 1%). Hepatotoxicity is likely due to hypersensitivity with primarily cholestatic patterns of liver enzymes, but mixed and hepatocellular patterns have been described. Our patient displayed a mixed pattern after one dose of Cefazolin, and eosinophils were present on biopsy consistent with hypersensitivity. This case highlights the importance of thoroughly reviewing a patient’s history to help diagnose potential causes of liver injury, especially DILI, as it is imperative to the definitive management.
Disclosures:
Patrick Lazarczyk, DO1, Eric M. Sieloff, MD1, Janice Fields, MD, FACG2. B0576 - It Only Takes One: A Case of Drug-Induced Liver Injury Due to Cefazolin Administration, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Ascension Providence Hospital, Southfield, MI; 2Ascension Providence, Southfield, MI
Introduction: Drug-induced liver injury (DILI) is a common cause of hepatotoxicity that has been associated with multiple medications and supplements. Herein, we present an intriguing case of DILI related to preoperative Cefazolin administration.
Case Description/Methods: A 64-year-old female with history of breast cancer presented for right upper quadrant abdominal pain, nausea, vomiting, and jaundice for 1 week. The patient underwent left mastectomy with administration of a single dose of preoperative IV Cefazolin about three weeks prior to presentation. On presentation, vital signs were within normal limits. On physical exam, the patient was noticeably jaundiced with right upper quadrant tenderness. Laboratory studies showed total bilirubin 11.9 mg/dL, direct bilirubin 8 mg/dL, alkaline phosphatase 603 IU/L, AST 165 IU/L, ALT 376 IU/L. Abdominal ultrasound found no biliary obstruction. Magnetic resonance cholangiopancreatography noted mild hepatomegaly with no intrahepatic or extrahepatic ductal dilatation. Workup for autoimmune disease, Wilson disease, and hemochromatosis was negative. Testing for hepatitis A and C was negative. The patient had positive HBcAb, however, HBsAg, HBsAb, and quantitative DNA PCR were negative. Liver biopsy showed cholestatic hepatitis, no significant fibrosis, and prominent eosinophils consistent with DILI. The patient was diagnosed with DILI secondary to Cefazolin administration. She was ultimately discharged home with improvement of symptoms and repeat lab work showing normalization of liver chemistries.
Discussion: Cefazolin is an antibiotic that is widely used for surgical prophylaxis, and it can be overlooked when working up acute liver injury, as it is usually a one-time dose. According to LiverTox, the latency period between administration and onset of liver injury is typically 1-4 weeks with most cases causing mild elevations of liver enzymes, however, significant elevations in aminotransferases greater than 5 times the upper limit of normal are rare (< 1%). Hepatotoxicity is likely due to hypersensitivity with primarily cholestatic patterns of liver enzymes, but mixed and hepatocellular patterns have been described. Our patient displayed a mixed pattern after one dose of Cefazolin, and eosinophils were present on biopsy consistent with hypersensitivity. This case highlights the importance of thoroughly reviewing a patient’s history to help diagnose potential causes of liver injury, especially DILI, as it is imperative to the definitive management.

Figure: Figure 1: H&E stain (high power) showing intrahepatic cholestasis (A). H&E stain (high power) showing portal inflammation with prominent eosinophils (B).
Disclosures:
Patrick Lazarczyk indicated no relevant financial relationships.
Eric Sieloff indicated no relevant financial relationships.
Janice Fields indicated no relevant financial relationships.
Patrick Lazarczyk, DO1, Eric M. Sieloff, MD1, Janice Fields, MD, FACG2. B0576 - It Only Takes One: A Case of Drug-Induced Liver Injury Due to Cefazolin Administration, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
