Back

Poster Session E - Tuesday Afternoon
Category: Colon
E0096 - Evaluation of Engraftment and Diversity Following Open-Label Administration of CP101, an Investigational Oral Microbiome Therapeutic for the Prevention of Recurrent C. difficile Infection, in the PRISM-EXT Trial
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Jessica R. Allegretti, MD, MPH
Brigham and Women’s Hospital Crohn’s and Colitis Center
Boston, MA
Presenting Author(s)
Jessica R. Allegretti, MD, MPH1, Colleen Kelly, MD, FACG2, Monika Fischer, MD, MSc3, Ylaine Gerardin, PhD4, Josh Silva, 4, Jennifer Lau, PhD4, Bharat Misra, MD5, Thomas J. Borody, MD, PhD, DSc, FACG6, Shrish Budree, MD4, Sahil Khanna, MBBS, MS, FACG7
1Brigham and Women’s Hospital Crohn’s and Colitis Center, Boston, MA; 2Warren Alpert Medical School of Brown University, Providence, RI; 3Indiana University, Indianapolis, IN; 4Finch Therapeutics, Somerville, MA; 5Borland Groover Clinic, Jacksonville, FL; 6Centre for Digestive Diseases, Five Dock, New South Wales, Australia; 7Mayo Clinic, Rochester, MN
Introduction: Disruption of the microbiome is key to the pathogenesis of recurrent Clostridioides difficile infection (CDI). CP101 is an investigational orally administered microbiome therapeutic designed to restore microbiome diversity and potentially enable early intervention in the management of recurrent CDI. The safety and efficacy profile of CP101 for the prevention of recurrent CDI has been evaluated in a Phase 2 placebo-controlled trial (PRISM3) and an open-label trial (PRISM-EXT). However, pharmacology data for investigational microbiome therapies, including engraftment of microbes and changes in microbial diversity remains limited.
Methods: PRISM-EXT enrolled participants with ≥1 CDI recurrences at 51 sites. The qualifying CDI episode was diagnosed by guideline-recommended testing (PCR or toxin EIA) and clinical symptoms. Following standard-of-care (SOC) antibiotics, participants received a one-time oral administration of CP101 without bowel preparation. The primary efficacy endpoint was the proportion of participants without CDI recurrence through Week 8. Exploratory microbiome endpoints were measured at baseline following SOC antibiotics, Week 8 and 24 using 16S rRNA gene amplicon sequencing. Engraftment of CP101-associated taxa was determined by identification of CP101-associated operational taxonomic units (OTUs) in participants’ post-treatment samples which were absent at baseline, as well as by global similarity between participants’ microbiome and CP101. Alpha diversity was measured using ecological richness, i.e., the number of unique OTUs per sample.
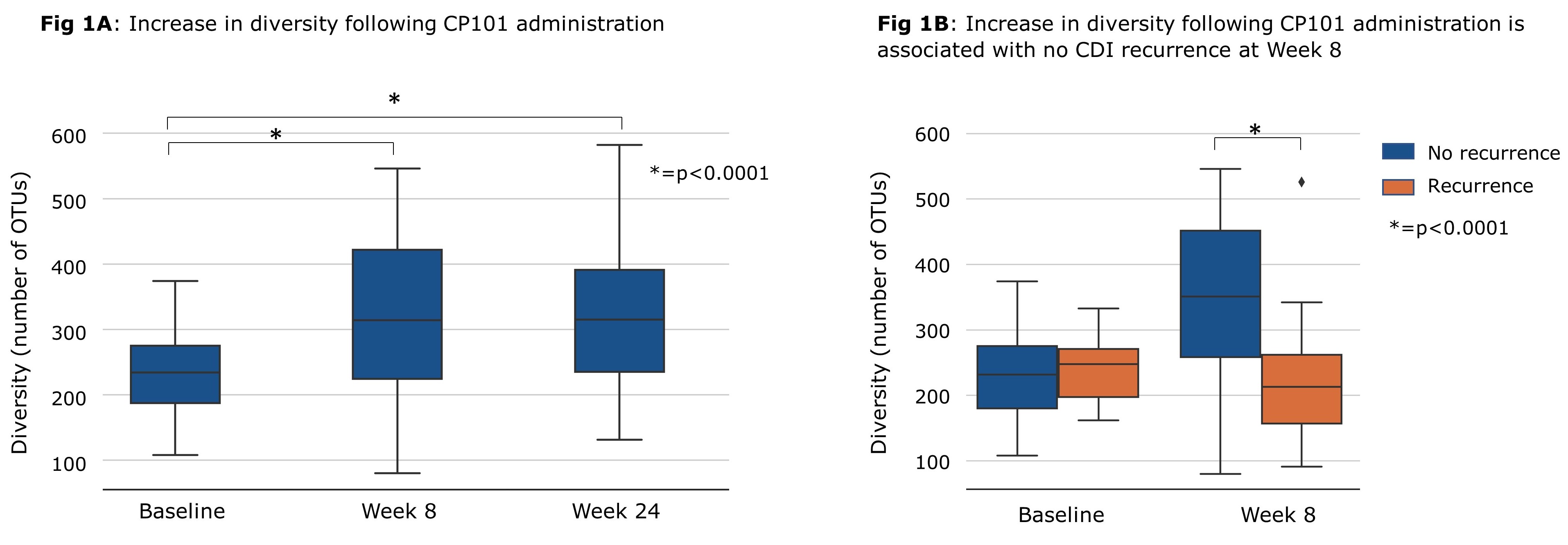
Results: Among the 132 PRISM-EXT participants, the proportion without CDI recurrence following administration of SOC antibiotics and CP101 was 80.3% through Week 8 and 78.8% through Week 24. No treatment-related serious adverse events were reported. Microbiome analysis showed that diversity significantly increased from baseline through Week 8 (p< 0.0001) and Week 24 (p< 0.0001, Fig 1A). Participant microbiomes became more similar to CP101 after treatment compared to baseline. Higher engraftment of CP101-associated taxa and improvement in diversity were both associated with prevention of CDI recurrence at Week 8 (Fig 1B).
Discussion: The majority of PRISM-EXT participants treated with CP101 had no CDI recurrence through Week 8 and 24. Microbiome analysis suggests that treatment with CP101 led to engraftment and an increase in gut microbiome diversity, both important factors that were associated with the prevention of recurrence.
Disclosures:
Jessica R. Allegretti, MD, MPH1, Colleen Kelly, MD, FACG2, Monika Fischer, MD, MSc3, Ylaine Gerardin, PhD4, Josh Silva, 4, Jennifer Lau, PhD4, Bharat Misra, MD5, Thomas J. Borody, MD, PhD, DSc, FACG6, Shrish Budree, MD4, Sahil Khanna, MBBS, MS, FACG7. E0096 - Evaluation of Engraftment and Diversity Following Open-Label Administration of CP101, an Investigational Oral Microbiome Therapeutic for the Prevention of Recurrent C. difficile Infection, in the PRISM-EXT Trial, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Brigham and Women’s Hospital Crohn’s and Colitis Center, Boston, MA; 2Warren Alpert Medical School of Brown University, Providence, RI; 3Indiana University, Indianapolis, IN; 4Finch Therapeutics, Somerville, MA; 5Borland Groover Clinic, Jacksonville, FL; 6Centre for Digestive Diseases, Five Dock, New South Wales, Australia; 7Mayo Clinic, Rochester, MN
Introduction: Disruption of the microbiome is key to the pathogenesis of recurrent Clostridioides difficile infection (CDI). CP101 is an investigational orally administered microbiome therapeutic designed to restore microbiome diversity and potentially enable early intervention in the management of recurrent CDI. The safety and efficacy profile of CP101 for the prevention of recurrent CDI has been evaluated in a Phase 2 placebo-controlled trial (PRISM3) and an open-label trial (PRISM-EXT). However, pharmacology data for investigational microbiome therapies, including engraftment of microbes and changes in microbial diversity remains limited.
Methods: PRISM-EXT enrolled participants with ≥1 CDI recurrences at 51 sites. The qualifying CDI episode was diagnosed by guideline-recommended testing (PCR or toxin EIA) and clinical symptoms. Following standard-of-care (SOC) antibiotics, participants received a one-time oral administration of CP101 without bowel preparation. The primary efficacy endpoint was the proportion of participants without CDI recurrence through Week 8. Exploratory microbiome endpoints were measured at baseline following SOC antibiotics, Week 8 and 24 using 16S rRNA gene amplicon sequencing. Engraftment of CP101-associated taxa was determined by identification of CP101-associated operational taxonomic units (OTUs) in participants’ post-treatment samples which were absent at baseline, as well as by global similarity between participants’ microbiome and CP101. Alpha diversity was measured using ecological richness, i.e., the number of unique OTUs per sample.
Results: Among the 132 PRISM-EXT participants, the proportion without CDI recurrence following administration of SOC antibiotics and CP101 was 80.3% through Week 8 and 78.8% through Week 24. No treatment-related serious adverse events were reported. Microbiome analysis showed that diversity significantly increased from baseline through Week 8 (p< 0.0001) and Week 24 (p< 0.0001, Fig 1A). Participant microbiomes became more similar to CP101 after treatment compared to baseline. Higher engraftment of CP101-associated taxa and improvement in diversity were both associated with prevention of CDI recurrence at Week 8 (Fig 1B).
Discussion: The majority of PRISM-EXT participants treated with CP101 had no CDI recurrence through Week 8 and 24. Microbiome analysis suggests that treatment with CP101 led to engraftment and an increase in gut microbiome diversity, both important factors that were associated with the prevention of recurrence.

Figure: Increase in diversity following CP101 administration is associated with no CDI recurrence at Week 8
Disclosures:
Jessica Allegretti: Artugen – Consultant. Baccain – Consultant. Bristol Myers Squibb – Consultant, Speaker. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant, Grant/Research Support. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Grant/Research Support. Morphic – Consultant. Pfizer – Consultant, Grant/Research Support. Seres Therapeutics – Consultant. Servatus – Consultant. Takeda – Consultant.
Colleen Kelly: Finch Therapeutics – Grant/Research Support. OpenBiome – Clinical Advisory Board.
Monika Fischer: AbbVie – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Rebiotix – Data and safety monitoring board. Scioto Biosciences – Advisory Committee/Board Member, Consultant.
Ylaine Gerardin: Finch Therapeutics – Employee, Stock Options.
Josh Silva: Finch Therapeutics – Employee.
Jennifer Lau: Finch Therapeutics – Employee, Stock-publicly held company(excluding mutual/index funds).
Bharat Misra: Finch Therapeutics – Grant/Research Support.
Thomas Borody: Axent medical Pty. Ltd – Advisory Committee/Board Member. Centre for Digestive Diseases – Advisory Committee/Board Member, Consultant, Employee, Grant/Research Support, Intellectual Property/Patents, Ownership interest, Owner/Ownership Interest, Stock-privately held company. Finch Therapeutics – Advisory Committee/Board Member, Intellectual Property/Patents, Stock-publicly held company(excluding mutual/index funds). Topelia Therapeutics – Advisor or Review Panel Member, Intellectual Property/Patents.
Shrish Budree: Finch Therapeutics – Employee, Stock-publicly held company(excluding mutual/index funds).
Sahil Khanna: Ferring Pharmaceuticals – Grant/Research Support. Finch – Grant/Research Support. Niche – Consultant. Pfizer – Grant/Research Support. Probiotech – Consultant. Seres Therapeutics – Grant/Research Support. Takeda/Shire – Consultant. Vedanta – Grant/Research Support.
Jessica R. Allegretti, MD, MPH1, Colleen Kelly, MD, FACG2, Monika Fischer, MD, MSc3, Ylaine Gerardin, PhD4, Josh Silva, 4, Jennifer Lau, PhD4, Bharat Misra, MD5, Thomas J. Borody, MD, PhD, DSc, FACG6, Shrish Budree, MD4, Sahil Khanna, MBBS, MS, FACG7. E0096 - Evaluation of Engraftment and Diversity Following Open-Label Administration of CP101, an Investigational Oral Microbiome Therapeutic for the Prevention of Recurrent C. difficile Infection, in the PRISM-EXT Trial, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
