Back

Poster Session A - Sunday Afternoon
Category: Stomach
A0686 - Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis in Study VP-VLY-686-3301
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Jesse L. Carlin, PhD
Vanda Pharmaceuticals, Inc.
Washington, DC
Presenting Author(s)
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD
Vanda Pharmaceuticals, Inc., Washington, DC
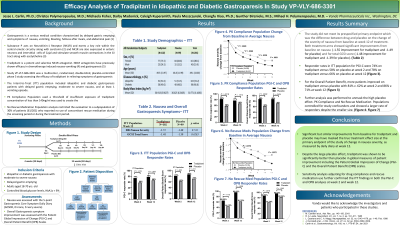
Introduction: This report presents the results of a multicenter, randomized, double-blind, placebo-controlled phase 3 study (VP-VLY-686-3301) assessing the efficacy of tradipitant, a novel NK-1 receptor antagonist, in relieving symptoms of gastroparesis
Methods: N=201 idiopathic and diabetic gastroparesis patients with delayed gastric emptying, moderate to severe nausea, and at least 1 vomiting episode were included in the ITT (Intent-to-Treat) population. Subjects were randomized to 85 mg tradipitant twice a day (n=102) or placebo (n=99) for 12 weeks. Nausea was assessed using a 5-point Gastroparesis Core Symptom Daily Diary (GCSDD). Overall gastroparesis symptom improvement was evaluated using the Patient Global Impression of Change (PGI-C) and the Overall Patient Benefit (OPB) scales. Sensitivity analyses were performed to control for confounders that may have masked true treatment effect size.
Results: At the primary endpoint of change in nausea from baseline as measured by the GCSDD at week 12, both tradipitant and placebo showed significant and similar reductions from baseline at 1.55 and 1.49 respectivelyand did not reach statistical significance. However, in the PGI-C scale, more tradipitant treated patients demonstrated response as compared to placebo both at week 2 (74% v. 58%, p=0.019) and at week 12 (78% v. 66%, p=0.065). Similarly, in the OPB scale, more tradipitant treated patients demonstrated response as compared to placebo both at week 2 (81% v. 62%, p=0.0003) and week 12 (86% v. 71%, p=0.011). Sensitivity analysis adjusting for PK compliance, rescue medication use, and baseline severity inflation further confirmed the ITT findings in both the PGI-C and OPB analysis at both week 2 and week 12. The large placebo effect observed may have been driven by Baseline Severity Inflation (BSI) especially on parameters which patients are selected for inclusion into the study. Tradipitant treatment in the BSI analysis subpopulation resulted in improvements in Nausea, PGI-C, and OPB Score both the earliest (Week 2) and last (Week 12) timepoints.
Discussion: Significant but similar improvements from baseline for tradipitant and placebo may have masked the true treatment effect size at the primary endpoint of the study of change in nausea severity as measured by daily diary at week 12 leading to no statistically significant difference between treatments
Disclosures:
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD. A0686 - Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis in Study VP-VLY-686-3301, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Vanda Pharmaceuticals, Inc., Washington, DC
Introduction: This report presents the results of a multicenter, randomized, double-blind, placebo-controlled phase 3 study (VP-VLY-686-3301) assessing the efficacy of tradipitant, a novel NK-1 receptor antagonist, in relieving symptoms of gastroparesis
Methods: N=201 idiopathic and diabetic gastroparesis patients with delayed gastric emptying, moderate to severe nausea, and at least 1 vomiting episode were included in the ITT (Intent-to-Treat) population. Subjects were randomized to 85 mg tradipitant twice a day (n=102) or placebo (n=99) for 12 weeks. Nausea was assessed using a 5-point Gastroparesis Core Symptom Daily Diary (GCSDD). Overall gastroparesis symptom improvement was evaluated using the Patient Global Impression of Change (PGI-C) and the Overall Patient Benefit (OPB) scales. Sensitivity analyses were performed to control for confounders that may have masked true treatment effect size.
Results: At the primary endpoint of change in nausea from baseline as measured by the GCSDD at week 12, both tradipitant and placebo showed significant and similar reductions from baseline at 1.55 and 1.49 respectivelyand did not reach statistical significance. However, in the PGI-C scale, more tradipitant treated patients demonstrated response as compared to placebo both at week 2 (74% v. 58%, p=0.019) and at week 12 (78% v. 66%, p=0.065). Similarly, in the OPB scale, more tradipitant treated patients demonstrated response as compared to placebo both at week 2 (81% v. 62%, p=0.0003) and week 12 (86% v. 71%, p=0.011). Sensitivity analysis adjusting for PK compliance, rescue medication use, and baseline severity inflation further confirmed the ITT findings in both the PGI-C and OPB analysis at both week 2 and week 12. The large placebo effect observed may have been driven by Baseline Severity Inflation (BSI) especially on parameters which patients are selected for inclusion into the study. Tradipitant treatment in the BSI analysis subpopulation resulted in improvements in Nausea, PGI-C, and OPB Score both the earliest (Week 2) and last (Week 12) timepoints.
Discussion: Significant but similar improvements from baseline for tradipitant and placebo may have masked the true treatment effect size at the primary endpoint of the study of change in nausea severity as measured by daily diary at week 12 leading to no statistically significant difference between treatments
Disclosures:
Jesse Carlin: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Christos Polymeropoulos: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Michaela Fisher: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Darby Madonick: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Caleigh Kupersmith: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Paula Moszczynski: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Changfu Xiao: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Gunther Birznieks: Vanda Pharmaceuticals, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Mihael Polymeropoulos: Vanda Pharmaceuticals, Inc. – Advisory Committee/Board Member, CEO, Stock-publicly held company(excluding mutual/index funds).
Jesse L. Carlin, PhD, Christos Polymeropoulos, MD, Michaela Fisher, BS, Darby Madonick, BA, Caleigh Kupersmith, BA, Paula Moszczynski, MS, Changfu Xiao, PhD, Gunther Birznieks, MS, Mihael H. Polymeropoulos, MD. A0686 - Efficacy Analysis of Tradipitant in Idiopathic and Diabetic Gastroparesis in Study VP-VLY-686-3301, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
