Back

Poster Session D - Tuesday Morning
Category: IBD
D0415 - Rapid Recurrence of Stroke After Tofacitinib Initiation: Composite Outcomes Challenge Informed Decision-Making in Ulcerative Colitis Management
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Matthew J. Townsend, MD, MSc, MPP
Duke University Medical Center
Durham, NC
Presenting Author(s)
Matthew J. Townsend, MD, MSc, MPP
Duke University Medical Center, Durham, NC
Introduction: Tofacitinib (Xeljanz) is an oral small molecule Janus kinase (JAK) inhibitor approved for the treatment of ulcerative colitis with incomplete response or intolerance to tumor necrosis factor (TNF) inhibitors. Since fall 2021 in the United States and Canada, tofacitinib carries a boxed warning for major adverse cardiovascular events (MACE) based on randomized, open-label study of cardiovascular risk-enriched adults. We present a case of recurrent stroke within one week of tofacitinib initiation in a patient with ulcerative colitis.
Case Description/Methods: A 59-year-old man with left-sided ulcerative colitis presented with acute-onset left hand weakness, numbness, facial droop, and slurred speech. He had a remote 4-pack year tobacco history and left-sided ischemic stroke 3 years ago in the setting of cocaine use. At that time, workup was unrevealing with normal cardiac function and no hyperlipidemia. He since reported complete abstinence from cocaine and continued on a statin and aspirin. His ulcerative colitis was diagnosed 8 months prior to presentation. He required multiple hospitalizations with inadequate response to oral mesalamine and intermittent intravenous steroids. He switched to infliximab but rapidly developed high anti-drug antibody titers. He started tofacitinib 10mg twice daily due to strong patient preference for an oral agent and concerns of developing antibodies against additional biologics. Five days after initiation, he presented with the neurologic symptoms above, which were mild and did not require tPA or thrombectomy. Computed tomography and magnetic resonance imaging revealed acute ischemic stroke in the posterior right frontal lobe. Low-density lipoprotein level was 40mg/dL; urine drug screen was negative. Expanded workup including hypercoagulability labs, echocardiogram, and 30-day cardiac loop monitor did not suggest a specific stroke etiology. He transitioned to ustekinumab.
Discussion: While tofacitinib has shown elevated MACE risk compared to TNF inhibitors in rheumatoid arthritis, no studies have yet examined these outcomes in ulcerative colitis or parsed risk of stroke from composite cardiovascular events. This case highlights the rapidity with which stroke can occur after tofacitinib initiation in ulcerative colitis in a patient with underlying risk factors. Non-composite outcome data are essential to guide shared decisionmaking using patient-specific risk factors. Evolving safety profiles must be carefully balanced against patient preferences for oral therapies.
Disclosures:
Matthew J. Townsend, MD, MSc, MPP. D0415 - Rapid Recurrence of Stroke After Tofacitinib Initiation: Composite Outcomes Challenge Informed Decision-Making in Ulcerative Colitis Management, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Duke University Medical Center, Durham, NC
Introduction: Tofacitinib (Xeljanz) is an oral small molecule Janus kinase (JAK) inhibitor approved for the treatment of ulcerative colitis with incomplete response or intolerance to tumor necrosis factor (TNF) inhibitors. Since fall 2021 in the United States and Canada, tofacitinib carries a boxed warning for major adverse cardiovascular events (MACE) based on randomized, open-label study of cardiovascular risk-enriched adults. We present a case of recurrent stroke within one week of tofacitinib initiation in a patient with ulcerative colitis.
Case Description/Methods: A 59-year-old man with left-sided ulcerative colitis presented with acute-onset left hand weakness, numbness, facial droop, and slurred speech. He had a remote 4-pack year tobacco history and left-sided ischemic stroke 3 years ago in the setting of cocaine use. At that time, workup was unrevealing with normal cardiac function and no hyperlipidemia. He since reported complete abstinence from cocaine and continued on a statin and aspirin. His ulcerative colitis was diagnosed 8 months prior to presentation. He required multiple hospitalizations with inadequate response to oral mesalamine and intermittent intravenous steroids. He switched to infliximab but rapidly developed high anti-drug antibody titers. He started tofacitinib 10mg twice daily due to strong patient preference for an oral agent and concerns of developing antibodies against additional biologics. Five days after initiation, he presented with the neurologic symptoms above, which were mild and did not require tPA or thrombectomy. Computed tomography and magnetic resonance imaging revealed acute ischemic stroke in the posterior right frontal lobe. Low-density lipoprotein level was 40mg/dL; urine drug screen was negative. Expanded workup including hypercoagulability labs, echocardiogram, and 30-day cardiac loop monitor did not suggest a specific stroke etiology. He transitioned to ustekinumab.
Discussion: While tofacitinib has shown elevated MACE risk compared to TNF inhibitors in rheumatoid arthritis, no studies have yet examined these outcomes in ulcerative colitis or parsed risk of stroke from composite cardiovascular events. This case highlights the rapidity with which stroke can occur after tofacitinib initiation in ulcerative colitis in a patient with underlying risk factors. Non-composite outcome data are essential to guide shared decisionmaking using patient-specific risk factors. Evolving safety profiles must be carefully balanced against patient preferences for oral therapies.

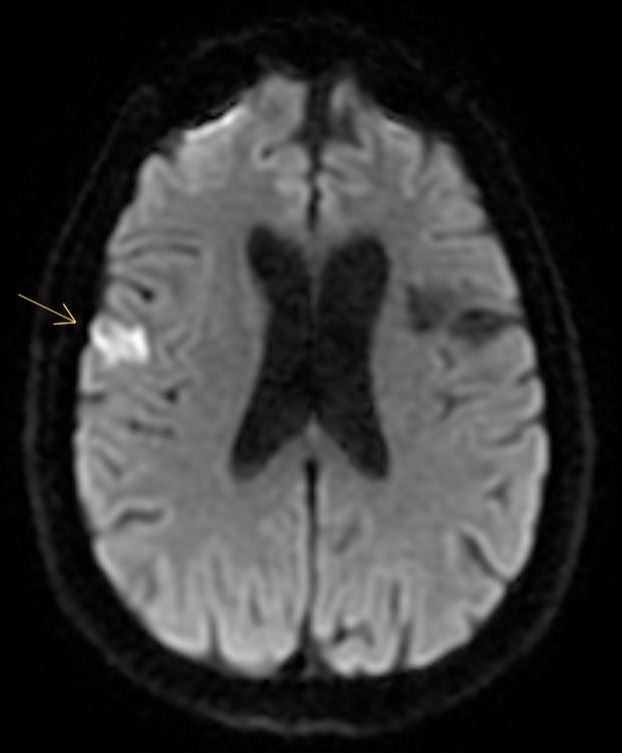
Figure: Figure 1. Recurrent acute ischemic stroke within one week of tofacitinib initiation. Magnetic resonance imaging reveals a focus of acute restricted diffusion (approximately 1.4 x 1.8 cm) within the posterior right frontal lobe (middle cerebral artery territory).
Disclosures:
Matthew Townsend indicated no relevant financial relationships.
Matthew J. Townsend, MD, MSc, MPP. D0415 - Rapid Recurrence of Stroke After Tofacitinib Initiation: Composite Outcomes Challenge Informed Decision-Making in Ulcerative Colitis Management, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
