Back


Poster Session E - Tuesday Afternoon
Category: Interventional Endoscopy
E0441 - Outcomes of Endoscopic Ultrasound-Guided Gallbladder Drainage in Malignant Biliary Obstruction: A Meta-Analysis
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Karim Osman, MD
Lahey Hospital & Medical Center
Burlington, MA
Presenting Author(s)
Ahmed Abdelfattah, MD1, Karim Osman, MD2, Prashanth Rau, MD3, Dhruval Amin, MD3, Sumayyah Akhtar, BS3, Neil Marya, MD3
1University of Massachusetts, Amherst, MA; 2Lahey Hospital & Medical Center, Burlington, MA; 3University of Massachusetts Medical Center, Worcester, MA
Introduction: Endoscopic ultrasound-guided gallbladder (EUS-GBD) drainage has been described as an alternative palliative treatment for malignant biliary obstruction (MBO). We aim to assess the outcomes of EUS-GBD for MBO.
Methods: We conducted a comprehensive literature review of MEDLINE, EMBASE, Cochrane, and Scopus databases for studies published in the English language that addressed outcomes of EUS-GBD for MBO through December 2021. Technical success was defined as successful stent deployment. Clinical success was defined as the resolution of the indication to proceed with EUS-GBD which includes improvement of jaundice or significant improvement of bilirubin. Reintervention was defined as the need for reintervention after achieving clinical success. Immediate adverse events (AE) were defined as complications that occurred intra-procedural till the first 24 hours after the procedure. Delayed AE were defined as complications that occurred after the first 24 hours of doing the EUS-GBD drainage. Pooled estimates were calculated following the restricted maximum likelihood method using random effects model. We assessed heterogeneity using the I2 statistic.
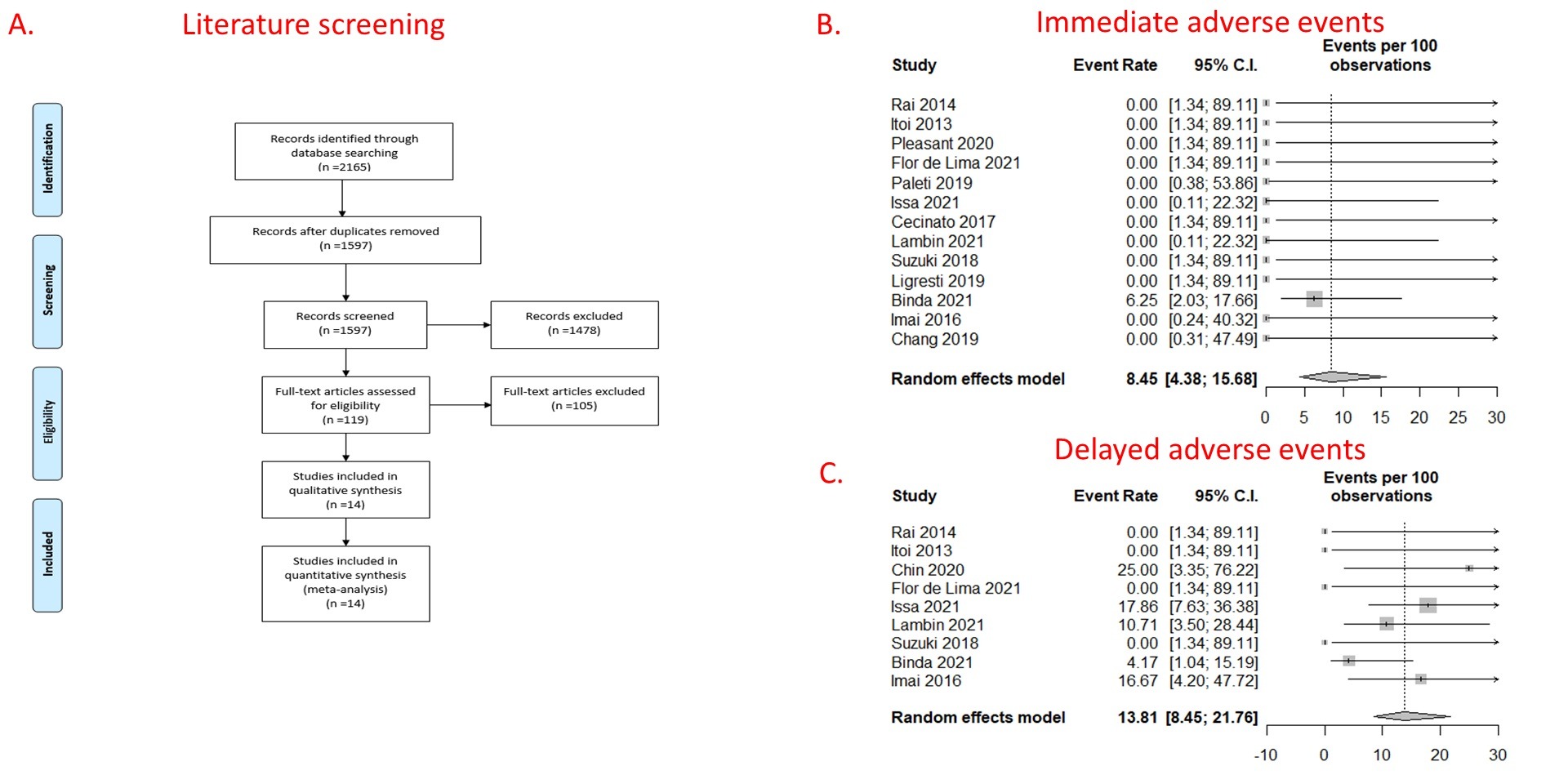
Results: After excluding duplicates, 1597 articles were screened with 14 unique articles included in the meta-analysis (Fig 1A). A total of 143 patients were included (Table). All studies had reported the technical success. The pooled technical success was 91.66% [95% confidence interval (CI) 83.13 - 96.08%]. Thirteen articles (139 patients) reported the clinical success and immediate AE. The pooled clinical success rate was 82.32% (95% CI 74.90-87.89%). The pooled immediate AE rates were 8.45% (95% CI 4.38-15.68) (Fig 1B). 9 articles (124 patients) reported delayed AE. The delayed AE reported were food impaction in the stent (n=3), stent migration &/or dysfunction (n=3), bleeding (n=2), cholangitis (n=1), peritonitis (n=1), and unknown AE (n=3). The pooled delayed AE rates were 13.81% (95% CI 8.45-21.76%) (Fig 1C). Nine articles (82 patients) reported intervention rates. The pooled reintervention rates after achieving clinical success were 15.71% (95% CI 9.20-25.51%). I2=0 for all meta-analyses.
Discussion: This is the first meta-analysis to evaluate outcomes of EUS-GBD drainage in MBO. We report a high technical and clinical success come up with a relatively low reintervention and AE rates. There was no heterogeneity in our data. EUS-GBD drainage is a feasible palliative option in MBO in experienced centers.
Disclosures:
Ahmed Abdelfattah, MD1, Karim Osman, MD2, Prashanth Rau, MD3, Dhruval Amin, MD3, Sumayyah Akhtar, BS3, Neil Marya, MD3. E0441 - Outcomes of Endoscopic Ultrasound-Guided Gallbladder Drainage in Malignant Biliary Obstruction: A Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Massachusetts, Amherst, MA; 2Lahey Hospital & Medical Center, Burlington, MA; 3University of Massachusetts Medical Center, Worcester, MA
Introduction: Endoscopic ultrasound-guided gallbladder (EUS-GBD) drainage has been described as an alternative palliative treatment for malignant biliary obstruction (MBO). We aim to assess the outcomes of EUS-GBD for MBO.
Methods: We conducted a comprehensive literature review of MEDLINE, EMBASE, Cochrane, and Scopus databases for studies published in the English language that addressed outcomes of EUS-GBD for MBO through December 2021. Technical success was defined as successful stent deployment. Clinical success was defined as the resolution of the indication to proceed with EUS-GBD which includes improvement of jaundice or significant improvement of bilirubin. Reintervention was defined as the need for reintervention after achieving clinical success. Immediate adverse events (AE) were defined as complications that occurred intra-procedural till the first 24 hours after the procedure. Delayed AE were defined as complications that occurred after the first 24 hours of doing the EUS-GBD drainage. Pooled estimates were calculated following the restricted maximum likelihood method using random effects model. We assessed heterogeneity using the I2 statistic.
Results: After excluding duplicates, 1597 articles were screened with 14 unique articles included in the meta-analysis (Fig 1A). A total of 143 patients were included (Table). All studies had reported the technical success. The pooled technical success was 91.66% [95% confidence interval (CI) 83.13 - 96.08%]. Thirteen articles (139 patients) reported the clinical success and immediate AE. The pooled clinical success rate was 82.32% (95% CI 74.90-87.89%). The pooled immediate AE rates were 8.45% (95% CI 4.38-15.68) (Fig 1B). 9 articles (124 patients) reported delayed AE. The delayed AE reported were food impaction in the stent (n=3), stent migration &/or dysfunction (n=3), bleeding (n=2), cholangitis (n=1), peritonitis (n=1), and unknown AE (n=3). The pooled delayed AE rates were 13.81% (95% CI 8.45-21.76%) (Fig 1C). Nine articles (82 patients) reported intervention rates. The pooled reintervention rates after achieving clinical success were 15.71% (95% CI 9.20-25.51%). I2=0 for all meta-analyses.
Discussion: This is the first meta-analysis to evaluate outcomes of EUS-GBD drainage in MBO. We report a high technical and clinical success come up with a relatively low reintervention and AE rates. There was no heterogeneity in our data. EUS-GBD drainage is a feasible palliative option in MBO in experienced centers.

Figure: Forest plots depicting immediate and delayed adverse events
Disclosures:
Ahmed Abdelfattah indicated no relevant financial relationships.
Karim Osman indicated no relevant financial relationships.
Prashanth Rau indicated no relevant financial relationships.
Dhruval Amin indicated no relevant financial relationships.
Sumayyah Akhtar indicated no relevant financial relationships.
Neil Marya: Boston Scientific – Consultant.
Ahmed Abdelfattah, MD1, Karim Osman, MD2, Prashanth Rau, MD3, Dhruval Amin, MD3, Sumayyah Akhtar, BS3, Neil Marya, MD3. E0441 - Outcomes of Endoscopic Ultrasound-Guided Gallbladder Drainage in Malignant Biliary Obstruction: A Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

