Back


Poster Session E - Tuesday Afternoon
Category: Liver
E0576 - A Rare Case of Drug-Induced Liver Injury (DILI) Due to Valproate Hepatocellular Injury
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
Hope Baldwin, BS
Wayne State University School of Medicine
Detroit, Michigan
Presenting Author(s)
Hope Baldwin, BS1, Syed-Mohammed Jafri, MD2, Ahmed Elbanna, DO2
1Wayne State University School of Medicine, Detroit, MI; 2Henry Ford Health System, Detroit, MI
Introduction: Valproate is a widely-used antiepileptic drug which works by increasing gamma-aminobutyric acid (GABA) levels in the brain. We present a unique form of drug-induced liver injury (DILI) in a patient prescribed valproate for trigeminal neuralgia pain.
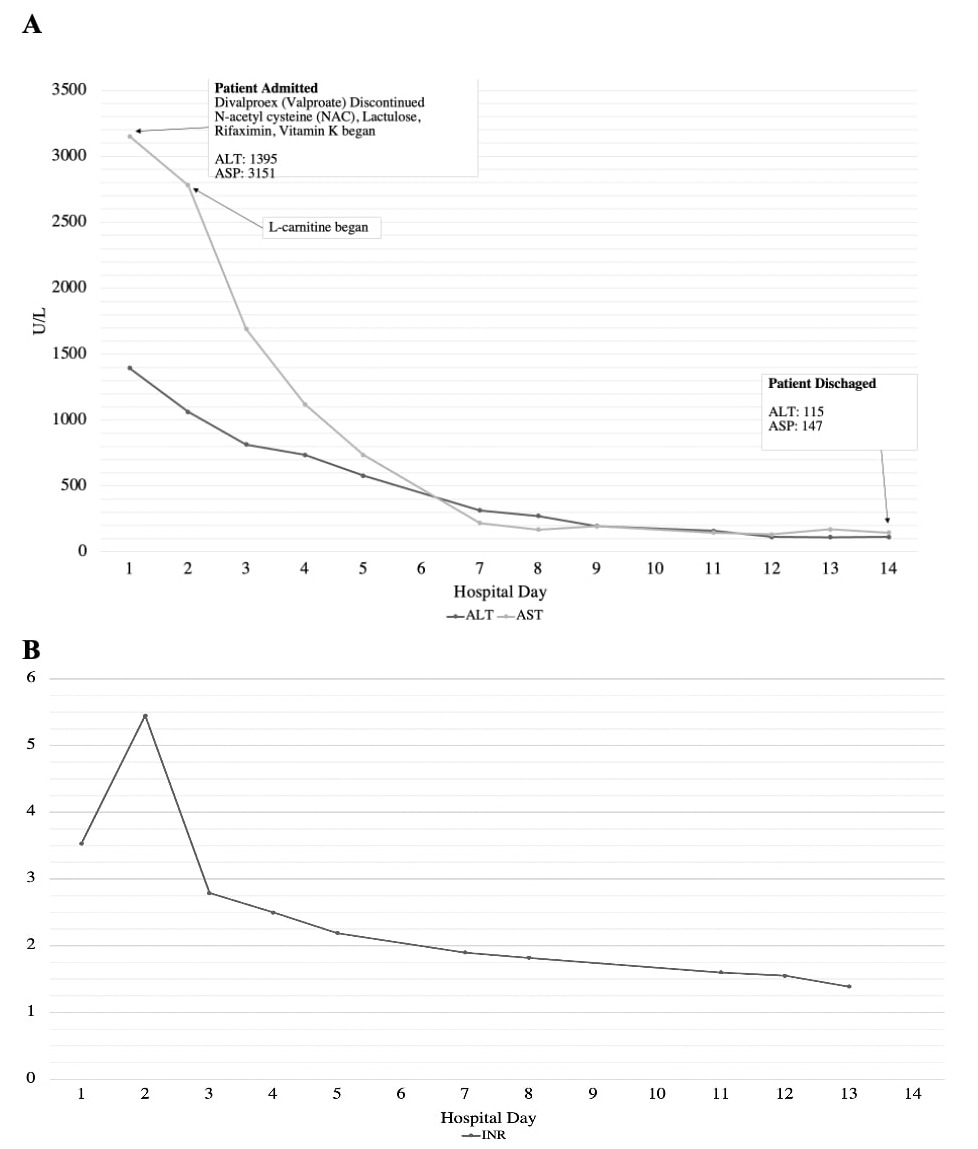
Case Description/Methods: A 45-year old African American female with a history of hypertension and trigeminal neuralgia was prescribed divalproex for trigeminal neuralgia pain after trials of duloxetine, gabapentin, baclofen, oxcarbazepine, carbamazepine, topiramate, amitriptyline, and tizanidine failed to provide relief. Six weeks later she presented with a two week history of worsening nausea, diarrhea, fever, facial rash, production of dark phlegm, and grade 1 encephalopathy. Lab results showed acute liver failure (ALF) with total bilirubin 15.6 mg/dL, ALP 180 U/L, AST 937 U/L, ALT 943 U/L, and INR 2.8. Exam findings included scleral icterus, hepatomegaly, small volume ascites, and some small liver cysts. A diagnosis of DILI was made, with likely drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Divalproex was discontinued and the patient was admitted for two weeks of stabilizing treatment including N-acetylcysteine, lactulose, rifaximin, Vitamin K, and L-carnitine transfusion (Figure 1). The patient was evaluated for liver transplant, but it was ultimately not indicated given her subsequent improvement in liver function. An acute kidney injury developed but was resolved before patient discharge.
Two weeks later the patient presented with signs of acute kidney injury. Upon readmission, complications including hepatic encephalopathy, macrocytic anemia, spontaneous bacterial peritonitis, and acute respiratory failure developed over the course of several weeks. All stabilizing treatment efforts were made but the patient ultimately developed septic shock and died.
Discussion: DILI is the most common cause of ALF in the United States. Valproate-induced hepatotoxicity as a result of mitochondrial injury from impaired beta-oxidation and decreased tissue carnitine levels is one rare effect of use. Such hepatocellular injury typically shows very elevated AST and ALT levels with a smaller elevation in ALP. Early diagnosis of DILI is crucial to promptly discontinue use of the causative agent and begin optimal treatment. In the case of valproate, it is recommended that patients receive intravenous (IV) carnitine until symptoms improve. Liver transplant evaluation is crucial for ALF patients given the high morality risk.
Disclosures:
Hope Baldwin, BS1, Syed-Mohammed Jafri, MD2, Ahmed Elbanna, DO2. E0576 - A Rare Case of Drug-Induced Liver Injury (DILI) Due to Valproate Hepatocellular Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Wayne State University School of Medicine, Detroit, MI; 2Henry Ford Health System, Detroit, MI
Introduction: Valproate is a widely-used antiepileptic drug which works by increasing gamma-aminobutyric acid (GABA) levels in the brain. We present a unique form of drug-induced liver injury (DILI) in a patient prescribed valproate for trigeminal neuralgia pain.
Case Description/Methods: A 45-year old African American female with a history of hypertension and trigeminal neuralgia was prescribed divalproex for trigeminal neuralgia pain after trials of duloxetine, gabapentin, baclofen, oxcarbazepine, carbamazepine, topiramate, amitriptyline, and tizanidine failed to provide relief. Six weeks later she presented with a two week history of worsening nausea, diarrhea, fever, facial rash, production of dark phlegm, and grade 1 encephalopathy. Lab results showed acute liver failure (ALF) with total bilirubin 15.6 mg/dL, ALP 180 U/L, AST 937 U/L, ALT 943 U/L, and INR 2.8. Exam findings included scleral icterus, hepatomegaly, small volume ascites, and some small liver cysts. A diagnosis of DILI was made, with likely drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Divalproex was discontinued and the patient was admitted for two weeks of stabilizing treatment including N-acetylcysteine, lactulose, rifaximin, Vitamin K, and L-carnitine transfusion (Figure 1). The patient was evaluated for liver transplant, but it was ultimately not indicated given her subsequent improvement in liver function. An acute kidney injury developed but was resolved before patient discharge.
Two weeks later the patient presented with signs of acute kidney injury. Upon readmission, complications including hepatic encephalopathy, macrocytic anemia, spontaneous bacterial peritonitis, and acute respiratory failure developed over the course of several weeks. All stabilizing treatment efforts were made but the patient ultimately developed septic shock and died.
Discussion: DILI is the most common cause of ALF in the United States. Valproate-induced hepatotoxicity as a result of mitochondrial injury from impaired beta-oxidation and decreased tissue carnitine levels is one rare effect of use. Such hepatocellular injury typically shows very elevated AST and ALT levels with a smaller elevation in ALP. Early diagnosis of DILI is crucial to promptly discontinue use of the causative agent and begin optimal treatment. In the case of valproate, it is recommended that patients receive intravenous (IV) carnitine until symptoms improve. Liver transplant evaluation is crucial for ALF patients given the high morality risk.

Figure: Figure 1A. Patient’s AST and ALT values over the course of her hospital admission. Figure 1B. Patient’s INR values over the course of her hospital admission.
Disclosures:
Hope Baldwin indicated no relevant financial relationships.
Syed-Mohammed Jafri indicated no relevant financial relationships.
Ahmed Elbanna indicated no relevant financial relationships.
Hope Baldwin, BS1, Syed-Mohammed Jafri, MD2, Ahmed Elbanna, DO2. E0576 - A Rare Case of Drug-Induced Liver Injury (DILI) Due to Valproate Hepatocellular Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
