Back

Poster Session A - Sunday Afternoon
Category: Liver
A0542 - COVID-19 Vaccine-Induced Liver Injury: A Case Series
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio
- GK
Gres Karim, MD
Mount Sinai Beth Israel
New York, NY
Presenting Author(s)
Award: Presidential Poster Award
Gres Karim, MD1, Nour Al Khalili, MD2, Minira Aslanova, DO1, Dewan Giri, MBBS1, Amreen Dinani, MD3, Ilan Weisberg, MD, MSc4
1Mount Sinai Beth Israel, New York, NY; 2Mount Sinai Morningside/West, New York, NY; 3Duke University, Durham, NC; 4New York-Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY
Introduction: The coronavirus 2019 (COVID-19) pandemic has caused over 248 million cases and over 5 million deaths worldwide. Given its high morbidity and mortality, there was a rapid effort to develop a vaccine within a year of the commencement of the pandemic. Although these vaccines have excellent safety profiles, there is a risk of adverse effects such as fever, fatigue, arthralgias, injection site pain, and, less commonly, anaphylactic reaction. Drug-induced hepatotoxicity (DIH) is a rare side effect of vaccines that has rarely been reported. We report a cohort of four patients who presented with DIH following COVID-19 vaccination.
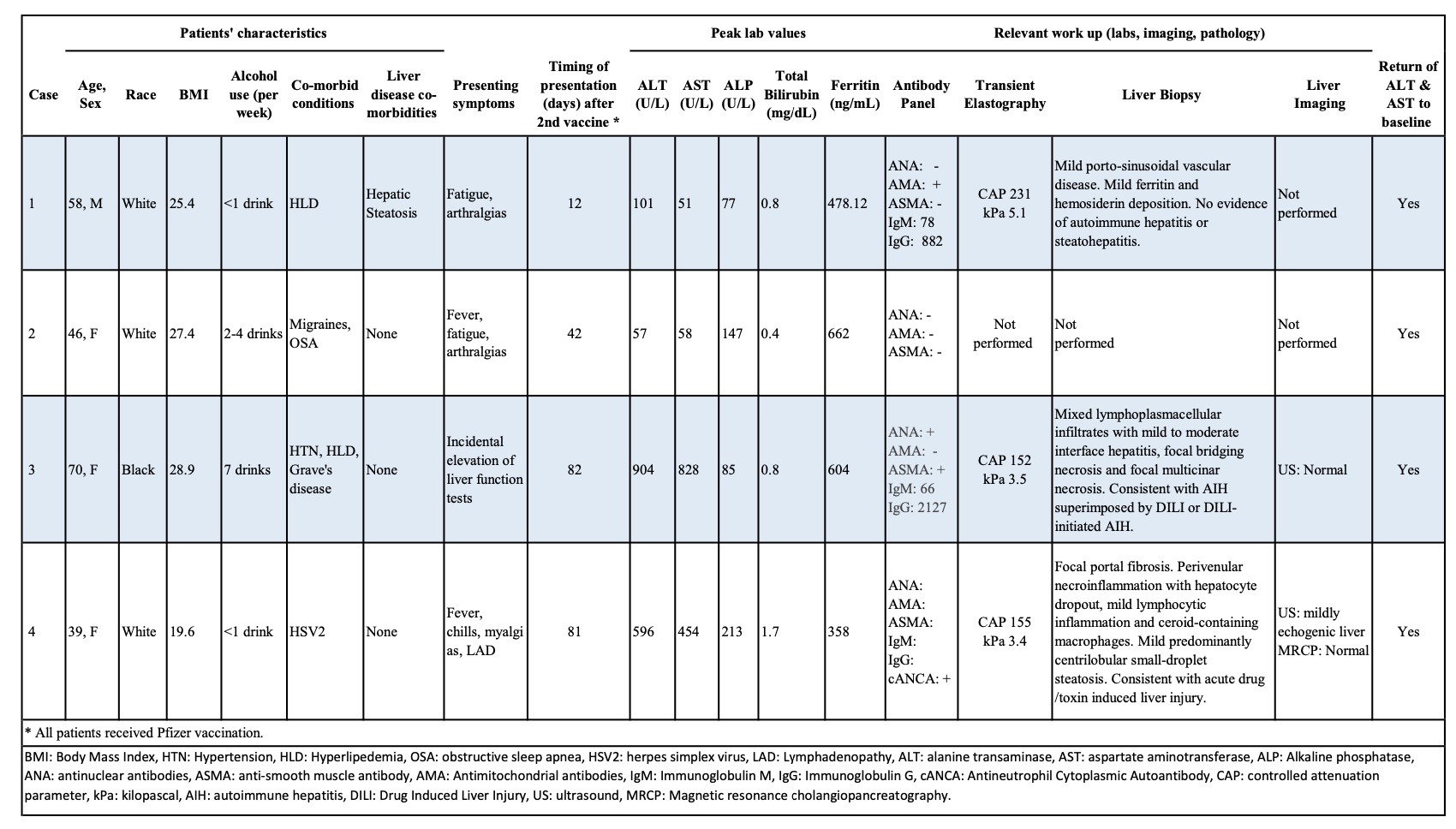
Case Description/Methods: Our cohort includes four patients, aged 39-70, who presented between 12 to 82 days after their second dose of Pfizer COVID-19 vaccine. Only one patient had a history of COVID-19 infection. One patient had a history of hepatic steatosis. All four patients had normal alanine transaminase [ALT] and aspartate transaminase [AST] within one year before the current presentation. All patients demonstrated a hepatocellular pattern of liver injury (peak ALT range of 57 to 904 U/L and AST range of 51 to 828 U/L). Tests for acute viral hepatitis were negative. One patient was found to have positive antinuclear antibodies and anti-smooth muscle antibodies. One patient was found to have an elevated antimitochondrial antibody. Liver stiffness measurement in three patients showed no evidence of fibrosis. Three patients underwent liver biopsy of which two suggested evidence of toxic/drug induced liver injury while the other demonstrated nonspecific inflammation.
Discussion: DIH leads to 10% of all cases of acute hepatitis and up to 50% of all cases of liver failure, making it one of the common reasons for withdrawal of medications from the market. We observed that our patients developed hepatic injury after Pfizer COVID-19 vaccination. Vaccine-induced immune-mediated hepatitis is a known phenomenon thought to be secondary to the COVID-19 spike protein triggering an auto-immune-like hepatic condition. This could explain the findings seen in our patients, raising the question of inflammatory response in patients with underlying autoimmune conditions. It is important that these patients receive pre and post vaccination laboratory monitoring, especially given the emergence of booster vaccinations. There is a need to follow these patients in the long-term to monitor for changes in clinical and laboratory studies in order to assess the risk of complications and outcomes.
Disclosures:
Gres Karim, MD1, Nour Al Khalili, MD2, Minira Aslanova, DO1, Dewan Giri, MBBS1, Amreen Dinani, MD3, Ilan Weisberg, MD, MSc4. A0542 - COVID-19 Vaccine-Induced Liver Injury: A Case Series, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Gres Karim, MD1, Nour Al Khalili, MD2, Minira Aslanova, DO1, Dewan Giri, MBBS1, Amreen Dinani, MD3, Ilan Weisberg, MD, MSc4
1Mount Sinai Beth Israel, New York, NY; 2Mount Sinai Morningside/West, New York, NY; 3Duke University, Durham, NC; 4New York-Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY
Introduction: The coronavirus 2019 (COVID-19) pandemic has caused over 248 million cases and over 5 million deaths worldwide. Given its high morbidity and mortality, there was a rapid effort to develop a vaccine within a year of the commencement of the pandemic. Although these vaccines have excellent safety profiles, there is a risk of adverse effects such as fever, fatigue, arthralgias, injection site pain, and, less commonly, anaphylactic reaction. Drug-induced hepatotoxicity (DIH) is a rare side effect of vaccines that has rarely been reported. We report a cohort of four patients who presented with DIH following COVID-19 vaccination.
Case Description/Methods: Our cohort includes four patients, aged 39-70, who presented between 12 to 82 days after their second dose of Pfizer COVID-19 vaccine. Only one patient had a history of COVID-19 infection. One patient had a history of hepatic steatosis. All four patients had normal alanine transaminase [ALT] and aspartate transaminase [AST] within one year before the current presentation. All patients demonstrated a hepatocellular pattern of liver injury (peak ALT range of 57 to 904 U/L and AST range of 51 to 828 U/L). Tests for acute viral hepatitis were negative. One patient was found to have positive antinuclear antibodies and anti-smooth muscle antibodies. One patient was found to have an elevated antimitochondrial antibody. Liver stiffness measurement in three patients showed no evidence of fibrosis. Three patients underwent liver biopsy of which two suggested evidence of toxic/drug induced liver injury while the other demonstrated nonspecific inflammation.
Discussion: DIH leads to 10% of all cases of acute hepatitis and up to 50% of all cases of liver failure, making it one of the common reasons for withdrawal of medications from the market. We observed that our patients developed hepatic injury after Pfizer COVID-19 vaccination. Vaccine-induced immune-mediated hepatitis is a known phenomenon thought to be secondary to the COVID-19 spike protein triggering an auto-immune-like hepatic condition. This could explain the findings seen in our patients, raising the question of inflammatory response in patients with underlying autoimmune conditions. It is important that these patients receive pre and post vaccination laboratory monitoring, especially given the emergence of booster vaccinations. There is a need to follow these patients in the long-term to monitor for changes in clinical and laboratory studies in order to assess the risk of complications and outcomes.

Figure: Patient demographics and characteristics
Disclosures:
Gres Karim indicated no relevant financial relationships.
Nour Al Khalili indicated no relevant financial relationships.
Minira Aslanova indicated no relevant financial relationships.
Dewan Giri indicated no relevant financial relationships.
Amreen Dinani indicated no relevant financial relationships.
Ilan Weisberg indicated no relevant financial relationships.
Gres Karim, MD1, Nour Al Khalili, MD2, Minira Aslanova, DO1, Dewan Giri, MBBS1, Amreen Dinani, MD3, Ilan Weisberg, MD, MSc4. A0542 - COVID-19 Vaccine-Induced Liver Injury: A Case Series, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

