Back
Poster Session E - Tuesday Afternoon
E0103 - Efficacy and Safety of Adipose-Derived Stem Cell Therapy for the Treatment of Complex Perianal Fistula Not Associated With Crohn’s Disease: A Systematic Review and Meta-Analysis
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom
.jpg)
Azizullah Beran, MD
University of Toledo
Toledo, OH
Presenting Author(s)
Azizullah Beran, MD1, Sami Ghazaleh, MD1, Wasef Sayeh, MD1, Mouhand F. Mohamed, MD, MSc2, Khaled Elfert, MD, MRCP3, Rami Musallam, MD4, Amna Iqbal, MD1, Syed Hamaad Rahman, DO5, Mohammad Safi, MD1, Muhammad Aziz, MD6, Ali Nawras, MD1
1University of Toledo, Toledo, OH; 2Warren Alpert Medical School of Brown University, Providence, RI; 3SBH Health System, New York, NY; 4St. Vincent Charity Medical Center, Cleveland, OH; 5Methodist Dallas Medical Center, Dallas, TX; 6The University of Toledo Medical Center, Toledo, OH
Introduction: Given the high recurrence rate and the risk of fecal incontinence with surgical options, Injection of adipose tissue-derived stem cells (ASC) has been arising as a novel method for treating complex perianal fistulas (CPAF). Therefore, we conducted a meta-analysis to evaluate the efficacy and safety of ASC in the management of CPAF not associated with Crohn’s disease.
Methods: We systematically searched Medline and Embase databases through April 20, 2022, for all studies that assessed the efficacy and safety of ASC for the treatment of CPAF not associated with Crohn’s disease. We excluded patients with rectovaginal fistulas and perianal fistulas associated with Crohn’s disease. Our primary outcome was the complete closure. The secondary outcomes included overall nonserious adverse events (NSAE), serious adverse events (SAE), and perianal abscess rate. All meta-analyses were conducted using a random-effect model. The publication bias was assessed by Egger’s test.
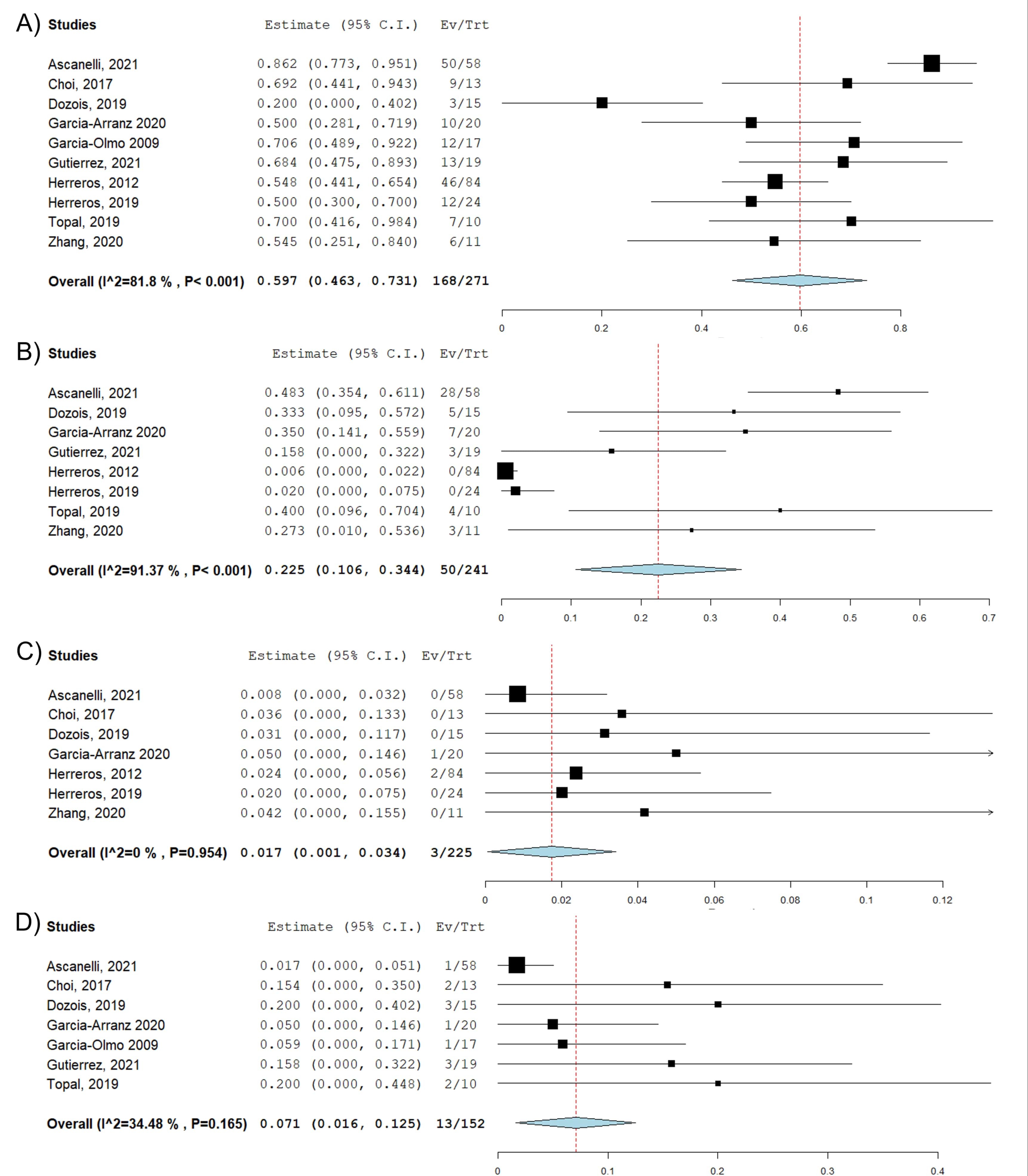
Results: Ten studies (eight clinical trials and two observational studies) with 271 patients were included in the pooled analysis. Eight studies used autologous stem cells, one used allogeneic stem cells, and one did not report the source of stem cells. The mean age of the patients was 43.7 years. The follow-up period ranged from 3 months to 2 years. The pooled complete closure rate was 59.7% (95% confidence interval (CI): 0.46-0.73, Figure 1A). On subgroup analysis based on country of origin, six studies with 213 patients were conducted in European countries, and four studies with 58 patients were conducted in non-European countries. The complete closure rate was higher in European countries than non-European countries, 64.1% vs. 52.6%. Eight studies reported overall NSAEs with the pooled NSAE rate of 22.5% (95% CI: 0.11-0.34, Figure 1B). Seven studies reported SAEs with the pooled SAE rate of 1.7% (95% CI: 0.001-0.034, Figure 1C). Seven studies reported the perianal abscess rate with a pooled perianal abscess rate of 7.1% (95% CI: 0.016-0.125, Figure 1D). No evidence of publication bias was found (Egger’s test: P=0.36).
Discussion: Our meta-analysis demonstrated that ASC is a promising therapeutic option for CPAF not associated with Crohn’s disease with a clinically adequate efficacy and low rate of adverse events. However, more studies with larger sample sizes are needed to provide a definitive assessment of the effectiveness of ASCs for CPAF not associated with Crohn’s disease.
Disclosures:
Azizullah Beran, MD1, Sami Ghazaleh, MD1, Wasef Sayeh, MD1, Mouhand F. Mohamed, MD, MSc2, Khaled Elfert, MD, MRCP3, Rami Musallam, MD4, Amna Iqbal, MD1, Syed Hamaad Rahman, DO5, Mohammad Safi, MD1, Muhammad Aziz, MD6, Ali Nawras, MD1. E0103 - Efficacy and Safety of Adipose-Derived Stem Cell Therapy for the Treatment of Complex Perianal Fistula Not Associated With Crohn’s Disease: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Toledo, Toledo, OH; 2Warren Alpert Medical School of Brown University, Providence, RI; 3SBH Health System, New York, NY; 4St. Vincent Charity Medical Center, Cleveland, OH; 5Methodist Dallas Medical Center, Dallas, TX; 6The University of Toledo Medical Center, Toledo, OH
Introduction: Given the high recurrence rate and the risk of fecal incontinence with surgical options, Injection of adipose tissue-derived stem cells (ASC) has been arising as a novel method for treating complex perianal fistulas (CPAF). Therefore, we conducted a meta-analysis to evaluate the efficacy and safety of ASC in the management of CPAF not associated with Crohn’s disease.
Methods: We systematically searched Medline and Embase databases through April 20, 2022, for all studies that assessed the efficacy and safety of ASC for the treatment of CPAF not associated with Crohn’s disease. We excluded patients with rectovaginal fistulas and perianal fistulas associated with Crohn’s disease. Our primary outcome was the complete closure. The secondary outcomes included overall nonserious adverse events (NSAE), serious adverse events (SAE), and perianal abscess rate. All meta-analyses were conducted using a random-effect model. The publication bias was assessed by Egger’s test.
Results: Ten studies (eight clinical trials and two observational studies) with 271 patients were included in the pooled analysis. Eight studies used autologous stem cells, one used allogeneic stem cells, and one did not report the source of stem cells. The mean age of the patients was 43.7 years. The follow-up period ranged from 3 months to 2 years. The pooled complete closure rate was 59.7% (95% confidence interval (CI): 0.46-0.73, Figure 1A). On subgroup analysis based on country of origin, six studies with 213 patients were conducted in European countries, and four studies with 58 patients were conducted in non-European countries. The complete closure rate was higher in European countries than non-European countries, 64.1% vs. 52.6%. Eight studies reported overall NSAEs with the pooled NSAE rate of 22.5% (95% CI: 0.11-0.34, Figure 1B). Seven studies reported SAEs with the pooled SAE rate of 1.7% (95% CI: 0.001-0.034, Figure 1C). Seven studies reported the perianal abscess rate with a pooled perianal abscess rate of 7.1% (95% CI: 0.016-0.125, Figure 1D). No evidence of publication bias was found (Egger’s test: P=0.36).
Discussion: Our meta-analysis demonstrated that ASC is a promising therapeutic option for CPAF not associated with Crohn’s disease with a clinically adequate efficacy and low rate of adverse events. However, more studies with larger sample sizes are needed to provide a definitive assessment of the effectiveness of ASCs for CPAF not associated with Crohn’s disease.

Figure: Figure 1
Disclosures:
Azizullah Beran indicated no relevant financial relationships.
Sami Ghazaleh indicated no relevant financial relationships.
Wasef Sayeh indicated no relevant financial relationships.
Mouhand Mohamed indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Rami Musallam indicated no relevant financial relationships.
Amna Iqbal indicated no relevant financial relationships.
Syed Hamaad Rahman indicated no relevant financial relationships.
Mohammad Safi indicated no relevant financial relationships.
Muhammad Aziz indicated no relevant financial relationships.
Ali Nawras indicated no relevant financial relationships.
Azizullah Beran, MD1, Sami Ghazaleh, MD1, Wasef Sayeh, MD1, Mouhand F. Mohamed, MD, MSc2, Khaled Elfert, MD, MRCP3, Rami Musallam, MD4, Amna Iqbal, MD1, Syed Hamaad Rahman, DO5, Mohammad Safi, MD1, Muhammad Aziz, MD6, Ali Nawras, MD1. E0103 - Efficacy and Safety of Adipose-Derived Stem Cell Therapy for the Treatment of Complex Perianal Fistula Not Associated With Crohn’s Disease: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
