Back
Poster Session A - Sunday Afternoon
Category: IBD
A0360 - Hospitalization and Surgery Rates in Patients Awaiting Approval of Biologics or Small Molecules for Treating Inflammatory Bowel Disease (IBD)
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom


Sarah Glover, DO
University of Mississippi Medical Center
Jackson, MS
Presenting Author(s)
Adam Parker, MD1, Brandon Brousse, MD1, Benjamin C. Billingsley, DO1, Alexander Carlyle, MS1, Alexander Abadir, MD2, Bobby Owens, PharmD1, Pegah Hosseini-Carroll, MD1, Konstantinos Papamichail, MD, PhD3, Adam Cheifetz, MD3, Sarah Glover, DO1
1University of Mississippi Medical Center, Jackson, MS; 2Harvard Medical School, Boston, MA; 3Beth Israel Deaconess Medical Center, Boston, MA
Introduction: Advanced IBD therapies including biological agents (infliximab, adalimumab, ustekinumab, golimumab, certolizumab pegol, and vedolizumab) and oral inhibitors (tofacitinib and ozanimod) have become mainstays of treatment for moderate to severe Crohn’s disease and ulcerative colitis. Although providers have anecdotal evidence that delays in insurance approval for these treatments might result in adverse outcomes, the rate of hospitalization and surgery during the prior approval process have not been formally evaluated. This study was designed to assess the rate of IBD-related hospitalizations and surgeries in individuals awaiting prior authorization for their advanced IBD therapy.
Methods: To assess the impact of the prior authorization process on clinical outcomes, we obtained IRB approval to evaluate the charts of individuals with IBD treated at our institution between of 3/1/2019-12/31/2021. Our state does has not adopted universal Medicaid. During this period, we found 542 individuals who had been started on a biological agent or an oral inhibitor. Using a data collection tool developed in the Harvard system, we identified 182 patients in whom we had complete data set. A complete data set included demographic data, disease variables, past medication history, insurance status, date of decision for medication, date of prior authorization, date of intuition of therapy, and clinical outcomes during the prior authorization period.
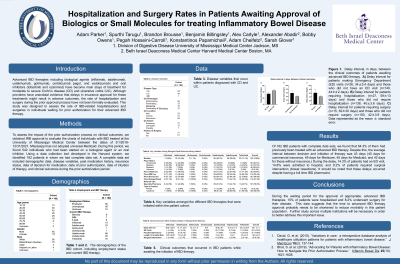
Results: Of 182 IBD patients with complete data sets, we found that 64.3% of them had previously been treated with an advanced IBD therapy. Despite this, the average interval between decision and initiation of therapy was 43 days (40 days for commercial insurance, 49 days for Medicare, 45 days for Medicaid, and 42 days for those without insurance.) During the delay, 14.3% of patients had an ED visit, 14.8% were admitted to hospital, and 8.2% of patients required surgical intervention (bowel resections). It should be noted that these delays occurred despite having a full time IBD pharmacist.
Discussion: During the waiting period for the approval of appropriate, advanced IBD therapies, 15% of patients were hospitalized and 8.2% underwent surgery for their disease. This data suggests that the time to advanced IBD therapy approval probably needs to be shortened so as to reduce morbidly in this patient population. Further study across multiple institutions will be necessary in order to better address this important issue.
Disclosures:
Adam Parker, MD1, Brandon Brousse, MD1, Benjamin C. Billingsley, DO1, Alexander Carlyle, MS1, Alexander Abadir, MD2, Bobby Owens, PharmD1, Pegah Hosseini-Carroll, MD1, Konstantinos Papamichail, MD, PhD3, Adam Cheifetz, MD3, Sarah Glover, DO1. A0360 - Hospitalization and Surgery Rates in Patients Awaiting Approval of Biologics or Small Molecules for Treating Inflammatory Bowel Disease (IBD), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Mississippi Medical Center, Jackson, MS; 2Harvard Medical School, Boston, MA; 3Beth Israel Deaconess Medical Center, Boston, MA
Introduction: Advanced IBD therapies including biological agents (infliximab, adalimumab, ustekinumab, golimumab, certolizumab pegol, and vedolizumab) and oral inhibitors (tofacitinib and ozanimod) have become mainstays of treatment for moderate to severe Crohn’s disease and ulcerative colitis. Although providers have anecdotal evidence that delays in insurance approval for these treatments might result in adverse outcomes, the rate of hospitalization and surgery during the prior approval process have not been formally evaluated. This study was designed to assess the rate of IBD-related hospitalizations and surgeries in individuals awaiting prior authorization for their advanced IBD therapy.
Methods: To assess the impact of the prior authorization process on clinical outcomes, we obtained IRB approval to evaluate the charts of individuals with IBD treated at our institution between of 3/1/2019-12/31/2021. Our state does has not adopted universal Medicaid. During this period, we found 542 individuals who had been started on a biological agent or an oral inhibitor. Using a data collection tool developed in the Harvard system, we identified 182 patients in whom we had complete data set. A complete data set included demographic data, disease variables, past medication history, insurance status, date of decision for medication, date of prior authorization, date of intuition of therapy, and clinical outcomes during the prior authorization period.
Results: Of 182 IBD patients with complete data sets, we found that 64.3% of them had previously been treated with an advanced IBD therapy. Despite this, the average interval between decision and initiation of therapy was 43 days (40 days for commercial insurance, 49 days for Medicare, 45 days for Medicaid, and 42 days for those without insurance.) During the delay, 14.3% of patients had an ED visit, 14.8% were admitted to hospital, and 8.2% of patients required surgical intervention (bowel resections). It should be noted that these delays occurred despite having a full time IBD pharmacist.
Discussion: During the waiting period for the approval of appropriate, advanced IBD therapies, 15% of patients were hospitalized and 8.2% underwent surgery for their disease. This data suggests that the time to advanced IBD therapy approval probably needs to be shortened so as to reduce morbidly in this patient population. Further study across multiple institutions will be necessary in order to better address this important issue.
Disclosures:
Adam Parker indicated no relevant financial relationships.
Brandon Brousse indicated no relevant financial relationships.
Benjamin Billingsley indicated no relevant financial relationships.
Alexander Carlyle indicated no relevant financial relationships.
Alexander Abadir indicated no relevant financial relationships.
Bobby Owens indicated no relevant financial relationships.
Pegah Hosseini-Carroll indicated no relevant financial relationships.
Konstantinos Papamichail indicated no relevant financial relationships.
Adam Cheifetz indicated no relevant financial relationships.
Sarah Glover: AbbVie – Speakers Bureau. BMS – Consultant. Celgene – Consultant. Janssen – Consultant. Takeda – Speakers Bureau.
Adam Parker, MD1, Brandon Brousse, MD1, Benjamin C. Billingsley, DO1, Alexander Carlyle, MS1, Alexander Abadir, MD2, Bobby Owens, PharmD1, Pegah Hosseini-Carroll, MD1, Konstantinos Papamichail, MD, PhD3, Adam Cheifetz, MD3, Sarah Glover, DO1. A0360 - Hospitalization and Surgery Rates in Patients Awaiting Approval of Biologics or Small Molecules for Treating Inflammatory Bowel Disease (IBD), ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

