Back

Poster Session A - Sunday Afternoon
Category: Colon
A0095 - Safety and Efficacy of Powered Non-Thermal Endoscopic Resection Device for Removal of Colonic Polyps: A Systematic Review and Meta-Analysis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio
.jpg)
Zohaib Ahmed, MD
University of Toledo
Maumee, OH
Presenting Author(s)
Zohaib Ahmed, MD, MPH1, Daryl Ramai, MD, MSc2, Umair Iqbal, MD3, Syeda F. Arif, 1, Wade M. Lee-Smith, MLS1, Joyce Badal, PharmD4, Ali Nawras, MD1, Yaseen Alastal, MD, MPH1, Harshit S. Khara, MD, FACG5, Bradley D. Confer, DO3, David L. Diehl, MD3, Douglas G. Adler, MD, FACG6
1University of Toledo, Toledo, OH; 2University of Utah, Salt Lake City, UT; 3Geisinger Medical Center, Danville, PA; 4University of Toledo College of Medicine, Toledo, OH; 5Geisinger Health System, Danville, PA; 6Centura Health-Porter Adventist Hospital, Salt Lake City, UT
Introduction: Endoscopic mucosal resection is a procedure commonly utilized for the resection of colonic polyps. However, polyp recurrence over a scarred submucosal base can make resection of residual lesions difficult using conventional techniques. EndoRotor is a non-thermal endoscopic mucosal resection device that has been recently evaluated in the resection of colonic polyps, non-dysplastic Barrett's esophagus, and pancreatic necrosis, but the studies are limited to small sample sizes. Therefore, we performed a systematic review and meta-analysis to evaluate the safety and efficacy of EndoRotor for the resection of colonic polyps.
Methods: A systematic review of the literature was performed using Medline, Embase, Web of Science, and the Cochrane library database until June 2022 to identify all studies that evaluated the safety of non-thermal endoscopic resection devices for the removal of colonic polyps. Our primary outcome of interest was the technical success rate, and secondary outcomes included rates of residual lesions and adverse events. All analyses were conducted using comprehensive meta-analysis software.
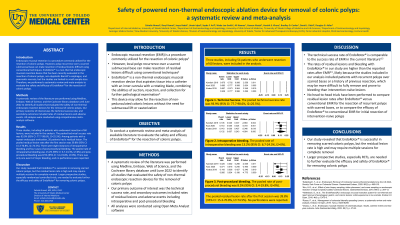
Results: Three studies, including 54 patients who underwent resection of 60 lesions, were included in the analysis. The pooled technical success rate was 93.9% (95% CI: 77.7-98.6%, I2=25.5%). Among patients with a repeat endoscopic evaluation, 20 patients had a residual lesion. The pooled residual lesion rate after the first session was 39.8% (95% CI: 15.3-70.8%, I2=74.5%). There were eight instances of intraoperative bleeding and four cases of post-procedural bleeding. The pooled rate of intraoperative bleeding was 13.2% (95% CI: 6.7-24.3%, I2=0%) and post-procedural bleeding was 8.5% (95% CI: 3.4-19.8%, I2=0%). There was only one event of major bleeding, and no perforations were reported.
Discussion: Our study revealed that EndoRotor is successful in removing scarred colonic polyps, but the residual lesion rate is high and may require multiple sessions for complete removal. Larger prospective studies, especially randomized controlled trials, are needed to evaluate further the efficacy and safety of EndoRotor for removing colonic polyps.
Disclosures:
Zohaib Ahmed, MD, MPH1, Daryl Ramai, MD, MSc2, Umair Iqbal, MD3, Syeda F. Arif, 1, Wade M. Lee-Smith, MLS1, Joyce Badal, PharmD4, Ali Nawras, MD1, Yaseen Alastal, MD, MPH1, Harshit S. Khara, MD, FACG5, Bradley D. Confer, DO3, David L. Diehl, MD3, Douglas G. Adler, MD, FACG6. A0095 - Safety and Efficacy of Powered Non-Thermal Endoscopic Resection Device for Removal of Colonic Polyps: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Toledo, Toledo, OH; 2University of Utah, Salt Lake City, UT; 3Geisinger Medical Center, Danville, PA; 4University of Toledo College of Medicine, Toledo, OH; 5Geisinger Health System, Danville, PA; 6Centura Health-Porter Adventist Hospital, Salt Lake City, UT
Introduction: Endoscopic mucosal resection is a procedure commonly utilized for the resection of colonic polyps. However, polyp recurrence over a scarred submucosal base can make resection of residual lesions difficult using conventional techniques. EndoRotor is a non-thermal endoscopic mucosal resection device that has been recently evaluated in the resection of colonic polyps, non-dysplastic Barrett's esophagus, and pancreatic necrosis, but the studies are limited to small sample sizes. Therefore, we performed a systematic review and meta-analysis to evaluate the safety and efficacy of EndoRotor for the resection of colonic polyps.
Methods: A systematic review of the literature was performed using Medline, Embase, Web of Science, and the Cochrane library database until June 2022 to identify all studies that evaluated the safety of non-thermal endoscopic resection devices for the removal of colonic polyps. Our primary outcome of interest was the technical success rate, and secondary outcomes included rates of residual lesions and adverse events. All analyses were conducted using comprehensive meta-analysis software.
Results: Three studies, including 54 patients who underwent resection of 60 lesions, were included in the analysis. The pooled technical success rate was 93.9% (95% CI: 77.7-98.6%, I2=25.5%). Among patients with a repeat endoscopic evaluation, 20 patients had a residual lesion. The pooled residual lesion rate after the first session was 39.8% (95% CI: 15.3-70.8%, I2=74.5%). There were eight instances of intraoperative bleeding and four cases of post-procedural bleeding. The pooled rate of intraoperative bleeding was 13.2% (95% CI: 6.7-24.3%, I2=0%) and post-procedural bleeding was 8.5% (95% CI: 3.4-19.8%, I2=0%). There was only one event of major bleeding, and no perforations were reported.
Discussion: Our study revealed that EndoRotor is successful in removing scarred colonic polyps, but the residual lesion rate is high and may require multiple sessions for complete removal. Larger prospective studies, especially randomized controlled trials, are needed to evaluate further the efficacy and safety of EndoRotor for removing colonic polyps.
Disclosures:
Zohaib Ahmed indicated no relevant financial relationships.
Daryl Ramai indicated no relevant financial relationships.
Umair Iqbal indicated no relevant financial relationships.
Syeda Arif indicated no relevant financial relationships.
Wade Lee-Smith indicated no relevant financial relationships.
Joyce Badal indicated no relevant financial relationships.
Ali Nawras indicated no relevant financial relationships.
Yaseen Alastal indicated no relevant financial relationships.
Harshit Khara indicated no relevant financial relationships.
Bradley Confer indicated no relevant financial relationships.
David Diehl: Acutuated Medical – Advisor or Review Panel Member. Boston Scientific – Advisor or Review Panel Member. Castle Biosciences – Advisor or Review Panel Member. GI-Supply – Advisor or Review Panel Member. Kite Endoscopic Innovations – Advisor or Review Panel Member. Lumendi – Advisor or Review Panel Member. Merit Medical – Advisor or Review Panel Member. Microtech – Advisor or Review Panel Member. Olympus – Advisor or Review Panel Member. One Pass Medical – Advisor or Review Panel Member. Pentax – Advisor or Review Panel Member. Steris – Advisor or Review Panel Member.
Douglas Adler: Boston Scientific – Consultant.
Zohaib Ahmed, MD, MPH1, Daryl Ramai, MD, MSc2, Umair Iqbal, MD3, Syeda F. Arif, 1, Wade M. Lee-Smith, MLS1, Joyce Badal, PharmD4, Ali Nawras, MD1, Yaseen Alastal, MD, MPH1, Harshit S. Khara, MD, FACG5, Bradley D. Confer, DO3, David L. Diehl, MD3, Douglas G. Adler, MD, FACG6. A0095 - Safety and Efficacy of Powered Non-Thermal Endoscopic Resection Device for Removal of Colonic Polyps: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
