Back

Poster Session A - Sunday Afternoon
Category: Liver
A0518 - Tenofovir Disoproxil Fumarate-Related Metabolic Syndrome and Steatohepatitis in a Case of Chronic Hepatitis B
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Nazli Begum Ozturk, MD
Istanbul University, Istanbul School of Medicine
Istanbul, Turkey
Presenting Author(s)
Nazli Begum Ozturk, MD1, Mehmet Akyuz, 2, Mine Gulluoglu, MD3, Filiz Akyuz, MD3
1Istanbul University, Istanbul School of Medicine, Rochester, MN; 2Yeditepe University School of Medicine, Istanbul, Istanbul, Turkey; 3Istanbul University, Istanbul School of Medicine, Istanbul, Istanbul, Turkey
Introduction: Tenofovir disoproxil fumarate (TDF) is a nucleotide analog used in the treatment of chronic hepatitis B virus infection (HBV). TDF is considered to have no direct hepatotoxicity. Herein we present a patient with HBV developing liver test abnormalities and biopsy-proven steatohepatitis (SH) after starting TDF, and the resolution of liver tests and SH after switching TDF to entecavir (ETV).
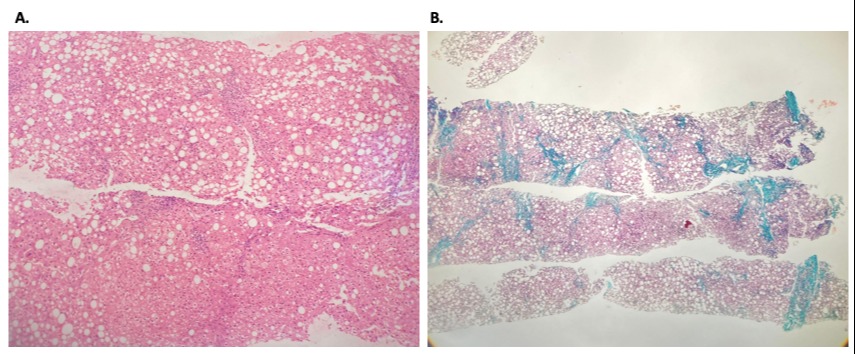
Case Description/Methods: A 48-year-old female patient was admitted to our clinic with positive HBsAg and HBV-DNA level of 21600 copies/mL. Her Anti-HBe was positive and Anti-HDV was negative. Abdominal ultrasonography revealed 2 cm hepatomegaly; her AST was 74 U/L (5-40 U/L) and ALT 125 U/L (10-40 U/L). Her BMI was within normal limits, she had no comorbidities and was not on any medication. Her lipid panel was within the normal range. She was prescribed 245 mg/day TDF, and was scheduled for regular clinical follow-up. Six months later, her AST was 113 U/L, ALT 148 U/L, and her HBV-DNA was negative. The patient was advised to follow-up closely. Eighteen months after the initiation of TDF, her AST increased to 211 U/L and ALT to 216 U/L. Her HbA1c was 6.1% and HOMA-IR was 4.0. MRI showed fat accumulation in the liver, and a liver needle biopsy was performed. Histological evaluation revealed grade 2 steatosis with moderate ballooning degeneration and stage 3/4 fibrosis (Figure 1). Autoimmune serologies including antinuclear antibody and anti-smooth muscle antibody were negative, and ceruloplasmin phenotype was normal. The causation of TDF was hypothesized. TDF was stopped, and ETV 0.5 mg/day was initiated. Her follow-up at month 3 revealed a decreased trend in transaminase levels. The patient lost to follow-up for 2 years, but she used ETV regularly. Her control HbA1c was 5.5% at this last visit and a control abdominal ultrasonography showed no fat accumulation.
Discussion: While it is known that TDF co-administration with other nucleoside analogs (e.g., didanosine, stavudine) can cause fatty infiltration of the liver, we present a rare case of metabolic syndrome and SH related with TDF monotherapy. Despite some reports suggesting tenofovir, specifically tenofovir alafenamide, might be associated with adverse metabolic changes in patients with HIV, our patient with HBV developed these changes while she was on TDF. We show that hepatic steatosis associated with TDF can be reversible with the medication change to ETV.
Disclosures:
Nazli Begum Ozturk, MD1, Mehmet Akyuz, 2, Mine Gulluoglu, MD3, Filiz Akyuz, MD3. A0518 - Tenofovir Disoproxil Fumarate-Related Metabolic Syndrome and Steatohepatitis in a Case of Chronic Hepatitis B, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Istanbul University, Istanbul School of Medicine, Rochester, MN; 2Yeditepe University School of Medicine, Istanbul, Istanbul, Turkey; 3Istanbul University, Istanbul School of Medicine, Istanbul, Istanbul, Turkey
Introduction: Tenofovir disoproxil fumarate (TDF) is a nucleotide analog used in the treatment of chronic hepatitis B virus infection (HBV). TDF is considered to have no direct hepatotoxicity. Herein we present a patient with HBV developing liver test abnormalities and biopsy-proven steatohepatitis (SH) after starting TDF, and the resolution of liver tests and SH after switching TDF to entecavir (ETV).
Case Description/Methods: A 48-year-old female patient was admitted to our clinic with positive HBsAg and HBV-DNA level of 21600 copies/mL. Her Anti-HBe was positive and Anti-HDV was negative. Abdominal ultrasonography revealed 2 cm hepatomegaly; her AST was 74 U/L (5-40 U/L) and ALT 125 U/L (10-40 U/L). Her BMI was within normal limits, she had no comorbidities and was not on any medication. Her lipid panel was within the normal range. She was prescribed 245 mg/day TDF, and was scheduled for regular clinical follow-up. Six months later, her AST was 113 U/L, ALT 148 U/L, and her HBV-DNA was negative. The patient was advised to follow-up closely. Eighteen months after the initiation of TDF, her AST increased to 211 U/L and ALT to 216 U/L. Her HbA1c was 6.1% and HOMA-IR was 4.0. MRI showed fat accumulation in the liver, and a liver needle biopsy was performed. Histological evaluation revealed grade 2 steatosis with moderate ballooning degeneration and stage 3/4 fibrosis (Figure 1). Autoimmune serologies including antinuclear antibody and anti-smooth muscle antibody were negative, and ceruloplasmin phenotype was normal. The causation of TDF was hypothesized. TDF was stopped, and ETV 0.5 mg/day was initiated. Her follow-up at month 3 revealed a decreased trend in transaminase levels. The patient lost to follow-up for 2 years, but she used ETV regularly. Her control HbA1c was 5.5% at this last visit and a control abdominal ultrasonography showed no fat accumulation.
Discussion: While it is known that TDF co-administration with other nucleoside analogs (e.g., didanosine, stavudine) can cause fatty infiltration of the liver, we present a rare case of metabolic syndrome and SH related with TDF monotherapy. Despite some reports suggesting tenofovir, specifically tenofovir alafenamide, might be associated with adverse metabolic changes in patients with HIV, our patient with HBV developed these changes while she was on TDF. We show that hepatic steatosis associated with TDF can be reversible with the medication change to ETV.

Figure: A: Hematoxylin-eosin staining shows 50% steatosis (grade 2), portal and lobular inflammation and moderate hepatocyte ballooning.
B: Masson-trichrome staining shows portocentral and pericellular fibrosis (stage 3/4).
B: Masson-trichrome staining shows portocentral and pericellular fibrosis (stage 3/4).
Disclosures:
Nazli Begum Ozturk indicated no relevant financial relationships.
Mehmet Akyuz indicated no relevant financial relationships.
Mine Gulluoglu indicated no relevant financial relationships.
Filiz Akyuz indicated no relevant financial relationships.
Nazli Begum Ozturk, MD1, Mehmet Akyuz, 2, Mine Gulluoglu, MD3, Filiz Akyuz, MD3. A0518 - Tenofovir Disoproxil Fumarate-Related Metabolic Syndrome and Steatohepatitis in a Case of Chronic Hepatitis B, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
