Back


Poster Session C - Monday Afternoon
Category: Interventional Endoscopy
C0461 - Assessment of Physical and Mental Health Before and After Protocolized Endoscopic Necrosectomy for Walled-Off Pancreatic Necrosis in a Prospective Multi-Center Trial
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Ambreen Anil Merchant, MD
Emory University
Dallas, TX
Presenting Author(s)
Ambreen Anil Merchant, MD1, Barham Abu Dayyeh, MD, MPH2, Vinay Chandrasekhara, MD2, Raj J. Shah, MD3, Jeffrey J. Easler, MD4, Andrew C. Storm, MD2, Mark D. Topazian, MD2, Michael J. Levy, MD2, John A. Martin, MD2, Bret T Petersen, MD2, Naoki Takahashi, MD2, Steven A. Edmundowicz, MD3, Mihir S. Wagh, MD, FACG5, Sachin Wani, MD6, John DeWitt, MD7, Mark A. Gromski, MD8, Mohammad Al-Haddad, MD, MSc8, Stuart Sherman, MD8, Joy Peetermans, PhD9, Ornela Gjata, MS10, Margaret Gourlay, MD, MPH9, Edmund McMullen, 11, Field F. Willingham, MD, MPH12
1Emory University, Dallas, TX; 2Mayo Clinic, Rochester, MN; 3University of Colorado Anschutz Medical Campus, Aurora, CO; 4Indiana University, Indianapolis, IN; 5University of Colorado, Aurora, CO; 6University of Colorado, Anschutz Medical Center, Aurora, CO; 7Indiana University Health Medical Center, Indianapolis, IN; 8Indiana University School of Medicine, Indianapolis, IN; 9Boston Scientific Corporation, Marlborough, MA; 10Boston Scientific Inc., Marlborough, MA; 11Boston Scientific, St. Paul, MN; 12Emory University School of Medicine, Atlanta, GA
Introduction: A regulatory FDA IDE prospective multi-center clinical trial (G170261-NCT03525808) was performed to evaluate rigorous protocolized management of walled off necrosis (WON) ≥ 6 cm in size with > 30% necrotic material using endoscopic ultrasound (EUS) guided lumen apposing metal stent (LAMS) placement with/without endoscopic necrosectomy. Few studies have examined changes in health related quality of life (HRQOL) before and after endoscopic management for WON. The SF-12 is a validated measure of HRQOL containing 12 items which yield a Mental Components Score (MCS) and a Physical Components Score (PCS). Higher scores correspond to a greater perceived quality of life.
Methods: The pre-specified target sample of 40 consecutive eligible patients was enrolled by multiple centers from September 2018 to March 2020. Patients with radiographic WON resolution (as demonstrated by size ≤ 3 cm on CT or MRI) and/or 60 days LAMS indwell time had stent removal followed by an end of study visit 6 months later. SF-12 quality of life questionnaires were completed by patients at baseline, stent removal and at the end of study visit.
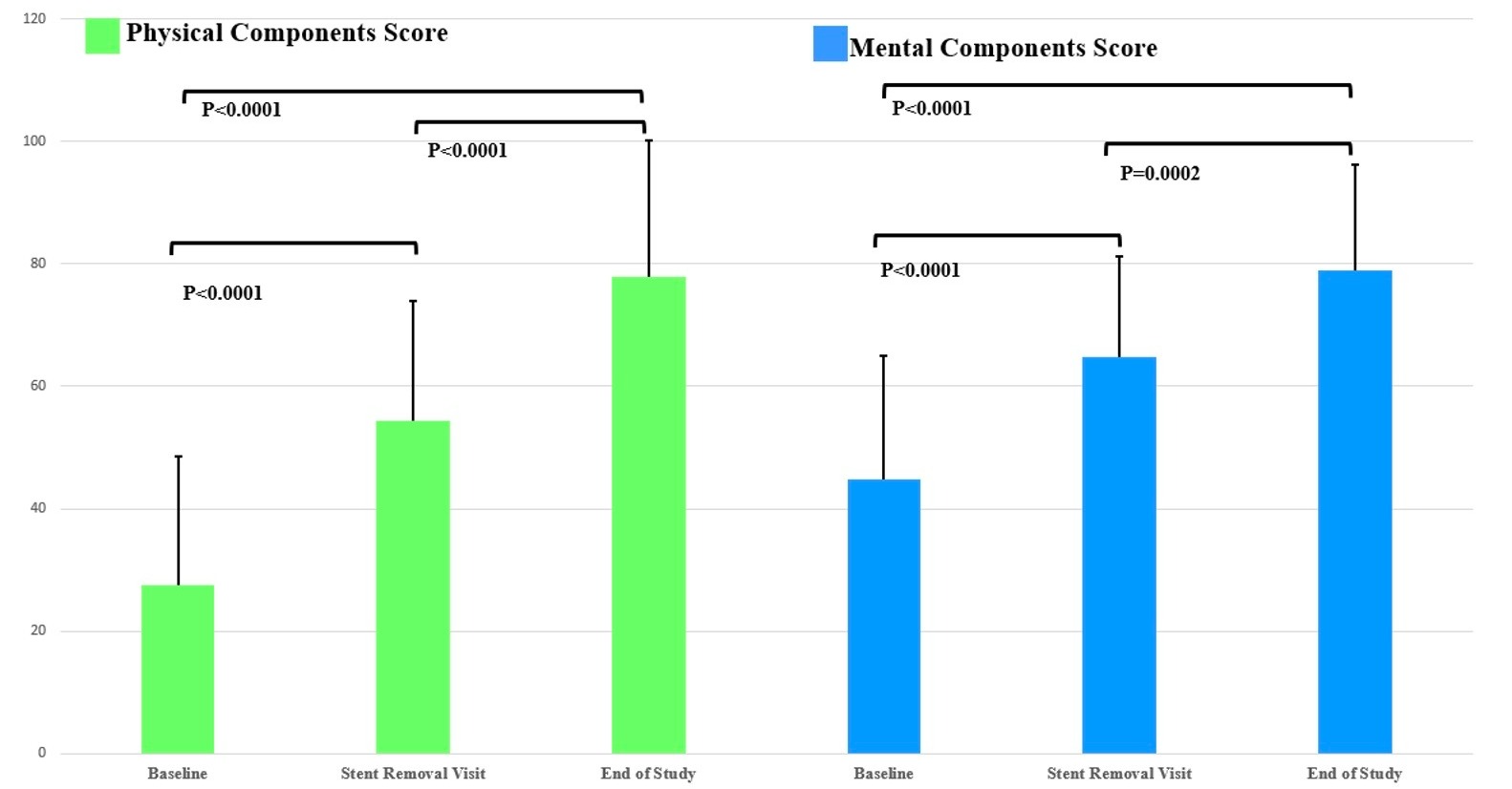
Results: Mean SF-12 Physical Components Score (PCS) was 27.5±21.1 at baseline, 54.4±19.5 at the time of LAMS removal, and 77.9±22.3 at end of study. Mean SF-12 Mental Components Score (MCS) was 44.9±20.1 at baseline, 64.7±16.5 at the time of LAMS removal, and 79.0±17.2 at end of study. The mean improvement in PCS score from baseline to LAMS removal was 26.0±24.8 (p< 0.0001), with the mean improvement in score from baseline to end of the study being 49.1±29.6 (p< 0.0001). The mean improvement in MCS was 19.6±20.1 from baseline to LAMS removal (p< 0.0001) and 32.0±22.3 from baseline to the end of study (p< 0.0001). The mean improvement from the time of stent removal to the end of study was 25.2±18.0 (PCS, p< 0.0001) and 15.5±18.5 (MCS, p=0.0002) (Figure 1).
Discussion: These data suggest that patients undergoing protocolized endoscopic necrosectomy for walled-off pancreatic necrosis experience significant improvement in both physical and mental HRQOL. Improvement in the physical score domain appeared to be greater than for the mental score domain. HRQOL indicators continued to improve over a mean follow-up of 180 days.

Disclosures:
Ambreen Anil Merchant, MD1, Barham Abu Dayyeh, MD, MPH2, Vinay Chandrasekhara, MD2, Raj J. Shah, MD3, Jeffrey J. Easler, MD4, Andrew C. Storm, MD2, Mark D. Topazian, MD2, Michael J. Levy, MD2, John A. Martin, MD2, Bret T Petersen, MD2, Naoki Takahashi, MD2, Steven A. Edmundowicz, MD3, Mihir S. Wagh, MD, FACG5, Sachin Wani, MD6, John DeWitt, MD7, Mark A. Gromski, MD8, Mohammad Al-Haddad, MD, MSc8, Stuart Sherman, MD8, Joy Peetermans, PhD9, Ornela Gjata, MS10, Margaret Gourlay, MD, MPH9, Edmund McMullen, 11, Field F. Willingham, MD, MPH12. C0461 - Assessment of Physical and Mental Health Before and After Protocolized Endoscopic Necrosectomy for Walled-Off Pancreatic Necrosis in a Prospective Multi-Center Trial, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Emory University, Dallas, TX; 2Mayo Clinic, Rochester, MN; 3University of Colorado Anschutz Medical Campus, Aurora, CO; 4Indiana University, Indianapolis, IN; 5University of Colorado, Aurora, CO; 6University of Colorado, Anschutz Medical Center, Aurora, CO; 7Indiana University Health Medical Center, Indianapolis, IN; 8Indiana University School of Medicine, Indianapolis, IN; 9Boston Scientific Corporation, Marlborough, MA; 10Boston Scientific Inc., Marlborough, MA; 11Boston Scientific, St. Paul, MN; 12Emory University School of Medicine, Atlanta, GA
Introduction: A regulatory FDA IDE prospective multi-center clinical trial (G170261-NCT03525808) was performed to evaluate rigorous protocolized management of walled off necrosis (WON) ≥ 6 cm in size with > 30% necrotic material using endoscopic ultrasound (EUS) guided lumen apposing metal stent (LAMS) placement with/without endoscopic necrosectomy. Few studies have examined changes in health related quality of life (HRQOL) before and after endoscopic management for WON. The SF-12 is a validated measure of HRQOL containing 12 items which yield a Mental Components Score (MCS) and a Physical Components Score (PCS). Higher scores correspond to a greater perceived quality of life.
Methods: The pre-specified target sample of 40 consecutive eligible patients was enrolled by multiple centers from September 2018 to March 2020. Patients with radiographic WON resolution (as demonstrated by size ≤ 3 cm on CT or MRI) and/or 60 days LAMS indwell time had stent removal followed by an end of study visit 6 months later. SF-12 quality of life questionnaires were completed by patients at baseline, stent removal and at the end of study visit.
Results: Mean SF-12 Physical Components Score (PCS) was 27.5±21.1 at baseline, 54.4±19.5 at the time of LAMS removal, and 77.9±22.3 at end of study. Mean SF-12 Mental Components Score (MCS) was 44.9±20.1 at baseline, 64.7±16.5 at the time of LAMS removal, and 79.0±17.2 at end of study. The mean improvement in PCS score from baseline to LAMS removal was 26.0±24.8 (p< 0.0001), with the mean improvement in score from baseline to end of the study being 49.1±29.6 (p< 0.0001). The mean improvement in MCS was 19.6±20.1 from baseline to LAMS removal (p< 0.0001) and 32.0±22.3 from baseline to the end of study (p< 0.0001). The mean improvement from the time of stent removal to the end of study was 25.2±18.0 (PCS, p< 0.0001) and 15.5±18.5 (MCS, p=0.0002) (Figure 1).
Discussion: These data suggest that patients undergoing protocolized endoscopic necrosectomy for walled-off pancreatic necrosis experience significant improvement in both physical and mental HRQOL. Improvement in the physical score domain appeared to be greater than for the mental score domain. HRQOL indicators continued to improve over a mean follow-up of 180 days.

Figure: Figure 1: SF-12 Physical and Mental Components Score
Table: Figure 1: SF-12 Physical and Mental Components Score
Disclosures:
Ambreen Anil Merchant indicated no relevant financial relationships.
Barham Abu Dayyeh: Apollo Endosurgery – Grant/Research Support. Aspire Bariatrics – Grant/Research Support. BFKW – Consultant. Boston Scientific – Consultant, Grant/Research Support. Cairn Diagnostics – Grant/Research Support. DyaMx – Consultant. Endogastric Solutions, – Speakers Bureau. Endo-TAGSS – Consultant. GI Dynamics – Grant/Research Support. Johnson and Johnson – Speakers Bureau. Medtronic; – Grant/Research Support. Metamodix – Consultant. Olympus – Speakers Bureau. Spatz Medical – Grant/Research Support. USGI Medical – Consultant, Grant/Research Support.
Vinay Chandrasekhara: Boston Scientific – Consultant. Covidien, LP – Consultant. Nevakar Corporation – Stock-privately held company.
Raj Shah: Boston Scientific – Consultant, Grant/Research Support.
Jeffrey Easler: Boston Scientific Corporation – Consultant, Grant/Research Support.
Andrew Storm: Apollo Endosurgery – Consultant. Apollo Endosurgery – Grant/Research Support. Boston Scientific – Grant/Research Support. Endo-TAGSS – Grant/Research Support. Enterasense – Consultant. ERBE – Consultant. GI Dynamics – Consultant. Olympus – Consultant.
Mark Topazian: Intuitive Surgical – Stock-publicly held company(excluding mutual/index funds). Quest Diagnostics – Stock-publicly held company(excluding mutual/index funds). Vertex Pharmaceuticals – Stock-publicly held company(excluding mutual/index funds).
Michael Levy indicated no relevant financial relationships.
John Martin indicated no relevant financial relationships.
Bret T Petersen: AMBU – Grant/Research Support. Boston scientific – Grant/Research Support. Olympus – Consultant.
Naoki Takahashi indicated no relevant financial relationships.
Steven Edmundowicz: Boston Scientific – Consultant. Motus GI – Stock-publicly held company(excluding mutual/index funds). Olympus – Advisor or Review Panel Member. Provation – Advisor or Review Panel Member.
Mihir Wagh: Allurion Technologies – Grant/Research Support. Boston Scientific – Consultant. ConMed – Consultant. Fujifilm – Consultant. Incyte – Consultant. Medtronic – Consultant. Olympus – Consultant. Steris/US Endoscopy – Grant/Research Support.
Sachin Wani: Ambu – Grant/Research Support. CDx Medical – Grant/Research Support. Cernostics – Consultant. Exact Sciences – Consultant. Lucid Medical – Grant/Research Support.
John DeWitt: Ariel Diagnostics – Consultant.
Mark Gromski: boston scientific – Consultant.
Mohammad Al-Haddad: Amplified sciences – Grant/Research Support. Cook endoscopy – Grant/Research Support. Creatics LLC – Grant/Research Support.
Stuart Sherman: Boston Scientific – Consultant.
Joy Peetermans: Boston Scientific Corporation – Employee, Stock Options.
Ornela Gjata indicated no relevant financial relationships.
Margaret Gourlay: Boston Scientific Corporation – Employee.
Edmund McMullen: Boston Scientific – Employee, Stock-publicly held company(excluding mutual/index funds).
Field Willingham: Boston Scientific – Grant/Research Support. Cancer Prevention Pharmaceuticals – Grant/Research Support. Cook Medical – Grant/Research Support. PCI Biotech – Grant/Research Support. Steris / CSA Medical – Grant/Research Support.
Ambreen Anil Merchant, MD1, Barham Abu Dayyeh, MD, MPH2, Vinay Chandrasekhara, MD2, Raj J. Shah, MD3, Jeffrey J. Easler, MD4, Andrew C. Storm, MD2, Mark D. Topazian, MD2, Michael J. Levy, MD2, John A. Martin, MD2, Bret T Petersen, MD2, Naoki Takahashi, MD2, Steven A. Edmundowicz, MD3, Mihir S. Wagh, MD, FACG5, Sachin Wani, MD6, John DeWitt, MD7, Mark A. Gromski, MD8, Mohammad Al-Haddad, MD, MSc8, Stuart Sherman, MD8, Joy Peetermans, PhD9, Ornela Gjata, MS10, Margaret Gourlay, MD, MPH9, Edmund McMullen, 11, Field F. Willingham, MD, MPH12. C0461 - Assessment of Physical and Mental Health Before and After Protocolized Endoscopic Necrosectomy for Walled-Off Pancreatic Necrosis in a Prospective Multi-Center Trial, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
