Back

Poster Session D - Tuesday Morning
Category: Colon
D0100 - Efficacy and Safety of RBX2660 in Patients After First Recurrence of Clostridioides difficile Infection – Results From a Phase 3, Randomized, Placebo-Controlled Study
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Sahil Khanna, MBBS, MS, FACG
Mayo Clinic
Rochester, MN
Presenting Author(s)
Sahil Khanna, MBBS, MS, FACG1, Glenn Tillotson, PhD2, Masakazu Ando, PhD3, Lindy Bancke, PharmD4, Adam Harvey, PhD4, Kevin W. Garey, PharmD, MS, FASHP, FIDSA, FCCP5, Kerry LaPlante, PharmD, FCCP, FIDSA, FIDP6
1Mayo Clinic, Rochester, MN; 2GST Micro, North, VA; 3Ferring Pharmaceuticals, Parsippany, NJ; 4Rebiotix Inc., a Ferring Company, Roseville, MN; 5University of Houston College of Pharmacy, Houston, TX; 6University of Rhode Island College of Pharmacy, Kingston, RI
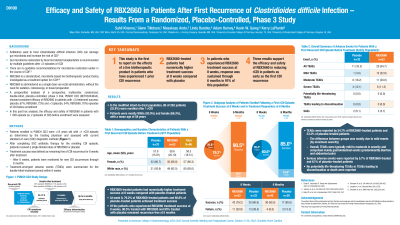
Introduction: Antibiotics used to treat Clostridioides difficile infection (CDI) can predispose patients to recurrent CDI (rCDI). Gut microbiome restoration by fecal microbiota transplantation is recommended by multiple guidelines after ≥2 rCDI, with no guideline recommended options to restore the microbiome earlier in the course of CDI. A prespecified analysis of a prospective, multicenter, randomized, double-blind, placebo-controlled phase 3 trial, PUNCH CD3 (NCT03244644), showed consistent efficacy of microbiota-based live biotherapeutic RBX2660 in participants with ≤3 (treatment success: placebo, 67%; RBX2660, 72%) and >3 (placebo, 54%; RBX2660, 70%) episodes of CDI prior to enrollment. In the present post-hoc analysis, we report the efficacy and safety of RBX2660 in a subgroup of patients with only 1 rCDI episode prior to enrollment.
Methods: Patients enrolled in PUNCH CD3 were ≥18 years old with ≥1 rCDI episode as determined by the treating physician and assessed with current standard-of-care (SOC) diagnostic methods. After completing SOC antibiotic therapy for the enrolling CDI episode, patients received a single blinded dose of RBX2660 or placebo. Treatment success was defined as remaining free of CDI recurrence for 8 weeks after treatment. Patients were monitored for recurrence through 6 months. Treatment-emergent adverse events (TEAEs) were summarized for the double-blind treatment period within 8 weeks.
Results: In the modified intent-to-treat population, 86 of 276 patients (32.2%) were enrolled after 1 rCDI. Patients were mostly white (93%) and female (66.3%) with a mean age of 58 years. At week 8, 79.2% (42/53) of RBX2660-treated patients and 60.6% (20/33) of placebo-treated patients achieved treatment success. Of responders, 90.5% (38/42) treated with RBX2660 and 85% (17/20) treated with placebo remained recurrence-free at 6 months. TEAEs were reported by 54.7% (29/53) of RBX2660-treated patients and 33.3% (11/33) of placebo-treated patients; mild events, mostly gastrointestinal, accounted for the difference. Serious adverse events were reported by 5.7% (3/53) of RBX2660-treated and 6.1% (2/33) of placebo-treated patients. No potentially life-threatening TEAEs or TEAEs leading to discontinuation or death were reported.
Discussion: After 1 rCDI episode, RBX2660-treated patients had numerically higher treatment success at week 8 and sustained response at 6 months compared to placebo. These results support the efficacy and safety of RBX2660 in reducing rCDI.
Disclosures:
Sahil Khanna, MBBS, MS, FACG1, Glenn Tillotson, PhD2, Masakazu Ando, PhD3, Lindy Bancke, PharmD4, Adam Harvey, PhD4, Kevin W. Garey, PharmD, MS, FASHP, FIDSA, FCCP5, Kerry LaPlante, PharmD, FCCP, FIDSA, FIDP6. D0100 - Efficacy and Safety of RBX2660 in Patients After First Recurrence of Clostridioides difficile Infection – Results From a Phase 3, Randomized, Placebo-Controlled Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2GST Micro, North, VA; 3Ferring Pharmaceuticals, Parsippany, NJ; 4Rebiotix Inc., a Ferring Company, Roseville, MN; 5University of Houston College of Pharmacy, Houston, TX; 6University of Rhode Island College of Pharmacy, Kingston, RI
Introduction: Antibiotics used to treat Clostridioides difficile infection (CDI) can predispose patients to recurrent CDI (rCDI). Gut microbiome restoration by fecal microbiota transplantation is recommended by multiple guidelines after ≥2 rCDI, with no guideline recommended options to restore the microbiome earlier in the course of CDI. A prespecified analysis of a prospective, multicenter, randomized, double-blind, placebo-controlled phase 3 trial, PUNCH CD3 (NCT03244644), showed consistent efficacy of microbiota-based live biotherapeutic RBX2660 in participants with ≤3 (treatment success: placebo, 67%; RBX2660, 72%) and >3 (placebo, 54%; RBX2660, 70%) episodes of CDI prior to enrollment. In the present post-hoc analysis, we report the efficacy and safety of RBX2660 in a subgroup of patients with only 1 rCDI episode prior to enrollment.
Methods: Patients enrolled in PUNCH CD3 were ≥18 years old with ≥1 rCDI episode as determined by the treating physician and assessed with current standard-of-care (SOC) diagnostic methods. After completing SOC antibiotic therapy for the enrolling CDI episode, patients received a single blinded dose of RBX2660 or placebo. Treatment success was defined as remaining free of CDI recurrence for 8 weeks after treatment. Patients were monitored for recurrence through 6 months. Treatment-emergent adverse events (TEAEs) were summarized for the double-blind treatment period within 8 weeks.
Results: In the modified intent-to-treat population, 86 of 276 patients (32.2%) were enrolled after 1 rCDI. Patients were mostly white (93%) and female (66.3%) with a mean age of 58 years. At week 8, 79.2% (42/53) of RBX2660-treated patients and 60.6% (20/33) of placebo-treated patients achieved treatment success. Of responders, 90.5% (38/42) treated with RBX2660 and 85% (17/20) treated with placebo remained recurrence-free at 6 months. TEAEs were reported by 54.7% (29/53) of RBX2660-treated patients and 33.3% (11/33) of placebo-treated patients; mild events, mostly gastrointestinal, accounted for the difference. Serious adverse events were reported by 5.7% (3/53) of RBX2660-treated and 6.1% (2/33) of placebo-treated patients. No potentially life-threatening TEAEs or TEAEs leading to discontinuation or death were reported.
Discussion: After 1 rCDI episode, RBX2660-treated patients had numerically higher treatment success at week 8 and sustained response at 6 months compared to placebo. These results support the efficacy and safety of RBX2660 in reducing rCDI.
Disclosures:
Sahil Khanna: Ferring Pharmaceuticals – Grant/Research Support. Finch – Grant/Research Support. Niche – Consultant. Pfizer – Grant/Research Support. Probiotech – Consultant. Seres Therapeutics – Grant/Research Support. Takeda/Shire – Consultant. Vedanta – Grant/Research Support.
Glenn Tillotson: Ferring Pharmaceuticals – Consultant. GST Micro LLC – Employee. Spero Pharmaceuticals – Consultant. Taro Pharmaceuticals – Consultant.
Masakazu Ando: Ferring Pharmaceuticals – Employee.
Lindy Bancke: Rebiotix Inc., a Ferring company – Employee.
Adam Harvey: Rebiotix Inc., a Ferring Company – Employee.
Kevin W. Garey: Acurx – Grant/Research Support. Ferring Pharmaceuticals – Consultant. Paratek Pharmaceuticals – Grant/Research Support. Seres Health – Grant/Research Support. Summit – Grant/Research Support.
Kerry LaPlante: Ferring Pharmaceuticals – Consultant.
Sahil Khanna, MBBS, MS, FACG1, Glenn Tillotson, PhD2, Masakazu Ando, PhD3, Lindy Bancke, PharmD4, Adam Harvey, PhD4, Kevin W. Garey, PharmD, MS, FASHP, FIDSA, FCCP5, Kerry LaPlante, PharmD, FCCP, FIDSA, FIDP6. D0100 - Efficacy and Safety of RBX2660 in Patients After First Recurrence of Clostridioides difficile Infection – Results From a Phase 3, Randomized, Placebo-Controlled Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
