Back

Poster Session D - Tuesday Morning
Category: Interventional Endoscopy
D0435 - Safety and Efficacy of the Novel EndoRotor Device for the Treatment of Walled-Off Pancreatic Necrosis (WOPN): A Systematic Review and Meta-Analysis
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Daryl Ramai, MD, MSc
University of Utah
Salt Lake City, UT
Presenting Author(s)
Daryl Ramai, MD, MSc1, Zohaib Ahmed, MD, MPH2, Smit Deliwala, MD3, Daniel Mozell, MD4, Antonio Facciorusso, MD, PhD5, Amanda Morgan, MD6, Andrea Anderloni, MD7, Monique Barakat, MD, PhD8, Yaseen Alastal, MD, MPH2, Douglas G. Adler, MD, FACG9
1University of Utah, Salt Lake City, UT; 2University of Toledo, Toledo, OH; 3Michigan State University at Hurley Medical Center, Flint, MI; 4Elmhurst Hospital, Elmhurst, NY; 5University of Foggia, Foggia, Puglia, Italy; 6Rocky Vista University, Ivins, UT; 7IRCCS Policlinico San Matteo, Pavia, Lombardia, Italy; 8Stanford University, Stanford, CA; 9Centura Health-Porter Adventist Hospital, Salt Lake City, UT
Introduction: Debridement of infected walled-off pancreatic necrosis (WOPN) is indicated to treat and prevent sepsis-related multi-organ failure. The lack of dedicated and effective accessories for WOPN debridement results in the need for time-consuming repetitive procedures. The aim of this study was to evaluate the efficacy and safety of the EndoRotor® powered endoscopic debridement system to remove solid debris under direct endoscopic visualization.
Methods: Search strategies were developed for PubMed, EMBASE, and Cochrane Library databases from inception through June 2022, in accordance with PRISMA and MOOSE guidelines. Outcomes of interest included technical success defined as successful use of device for debridement, clinical success defined as complete debridement and cyst resolution, and procedure-related adverse events. A random effects model was used for analysis and results were expressed as Odds ratio (OR) along with 95% confidence interval (CI).
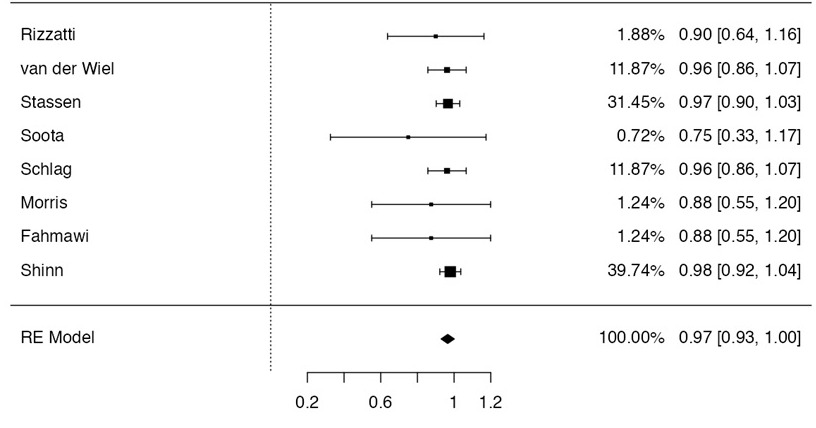
Results: A total of 8 studies (n = 91 patients) were included. The most common etiology of WOPN was biliary (30%). Mean WOPN size was 154.6 ± 34.0 mm, while mean procedure time was 71.4 minutes. LAMS were used to create a cystgastrostomy for 73.6% of cases with the remaining cases relying upon a placement of plastic stents (24.2%) and self-expanding metal stents (SEMS) (2.2%). The mean number of necrosectomy sessions required was 2.2 (range 1 to 7). The pooled rate of clinical success was 97% (95% CI 93-100%, I2 = 0%) with a pooled technical success rate of 97% (93-100%, I2 Z54%). The pooled procedure-related adverse event rate was 7% (2-12%, I2 = 0%), which included procedure-associated bleeding, pneumoperitoneum, peritonitis, pleural effusion, and dislodgement of LAMS. Overall publication bias was considered low.
Discussion: Our study illustrates that the novel EndoRotor device appears to be safe and effective for treating pancreatic necrosis. Patients undergoing endoscopic necrosectomy with the EndoRotor appear to require less debridement sessions when compared with studies using conventional instruments.
Disclosures:
Daryl Ramai, MD, MSc1, Zohaib Ahmed, MD, MPH2, Smit Deliwala, MD3, Daniel Mozell, MD4, Antonio Facciorusso, MD, PhD5, Amanda Morgan, MD6, Andrea Anderloni, MD7, Monique Barakat, MD, PhD8, Yaseen Alastal, MD, MPH2, Douglas G. Adler, MD, FACG9. D0435 - Safety and Efficacy of the Novel EndoRotor Device for the Treatment of Walled-Off Pancreatic Necrosis (WOPN): A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Utah, Salt Lake City, UT; 2University of Toledo, Toledo, OH; 3Michigan State University at Hurley Medical Center, Flint, MI; 4Elmhurst Hospital, Elmhurst, NY; 5University of Foggia, Foggia, Puglia, Italy; 6Rocky Vista University, Ivins, UT; 7IRCCS Policlinico San Matteo, Pavia, Lombardia, Italy; 8Stanford University, Stanford, CA; 9Centura Health-Porter Adventist Hospital, Salt Lake City, UT
Introduction: Debridement of infected walled-off pancreatic necrosis (WOPN) is indicated to treat and prevent sepsis-related multi-organ failure. The lack of dedicated and effective accessories for WOPN debridement results in the need for time-consuming repetitive procedures. The aim of this study was to evaluate the efficacy and safety of the EndoRotor® powered endoscopic debridement system to remove solid debris under direct endoscopic visualization.
Methods: Search strategies were developed for PubMed, EMBASE, and Cochrane Library databases from inception through June 2022, in accordance with PRISMA and MOOSE guidelines. Outcomes of interest included technical success defined as successful use of device for debridement, clinical success defined as complete debridement and cyst resolution, and procedure-related adverse events. A random effects model was used for analysis and results were expressed as Odds ratio (OR) along with 95% confidence interval (CI).
Results: A total of 8 studies (n = 91 patients) were included. The most common etiology of WOPN was biliary (30%). Mean WOPN size was 154.6 ± 34.0 mm, while mean procedure time was 71.4 minutes. LAMS were used to create a cystgastrostomy for 73.6% of cases with the remaining cases relying upon a placement of plastic stents (24.2%) and self-expanding metal stents (SEMS) (2.2%). The mean number of necrosectomy sessions required was 2.2 (range 1 to 7). The pooled rate of clinical success was 97% (95% CI 93-100%, I2 = 0%) with a pooled technical success rate of 97% (93-100%, I2 Z54%). The pooled procedure-related adverse event rate was 7% (2-12%, I2 = 0%), which included procedure-associated bleeding, pneumoperitoneum, peritonitis, pleural effusion, and dislodgement of LAMS. Overall publication bias was considered low.
Discussion: Our study illustrates that the novel EndoRotor device appears to be safe and effective for treating pancreatic necrosis. Patients undergoing endoscopic necrosectomy with the EndoRotor appear to require less debridement sessions when compared with studies using conventional instruments.

Figure: Forrest plot of pooled rates of clinical success.
Disclosures:
Daryl Ramai indicated no relevant financial relationships.
Zohaib Ahmed indicated no relevant financial relationships.
Smit Deliwala indicated no relevant financial relationships.
Daniel Mozell indicated no relevant financial relationships.
Antonio Facciorusso indicated no relevant financial relationships.
Amanda Morgan indicated no relevant financial relationships.
Andrea Anderloni indicated no relevant financial relationships.
Monique Barakat indicated no relevant financial relationships.
Yaseen Alastal indicated no relevant financial relationships.
Douglas Adler: Boston Scientific – Consultant.
Daryl Ramai, MD, MSc1, Zohaib Ahmed, MD, MPH2, Smit Deliwala, MD3, Daniel Mozell, MD4, Antonio Facciorusso, MD, PhD5, Amanda Morgan, MD6, Andrea Anderloni, MD7, Monique Barakat, MD, PhD8, Yaseen Alastal, MD, MPH2, Douglas G. Adler, MD, FACG9. D0435 - Safety and Efficacy of the Novel EndoRotor Device for the Treatment of Walled-Off Pancreatic Necrosis (WOPN): A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
