Back

Poster Session D - Tuesday Morning
Category: Liver
D0580 - Delayed Onset Drug-Induced Acute Liver Failure Caused by Glatiramer Acetate (GA) in Multiple Sclerosis Requiring Liver Transplantation
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
.jpg)
Diep Edwards, MD
Johns Hopkins School of Medicine
Baltimore, MD
Presenting Author(s)
Diep Edwards, MD, Christine Lin, BS, Jessica Lin, MD, Kiyoko Oshima, MD, Elizabeth King, MD, Russell Wesson, MD, Peng-sheng Ting, MD, Shane Ottmann, MD, Ahmet Gurakar, MD
Johns Hopkins School of Medicine, Baltimore, MD
Introduction: Glatiramer acetate (GA) has been used for multiple sclerosis (MS) since 1996. Regular liver function test monitoring is not required for the medication because there have not been any reported cases of liver toxicity.1Here, we report the first case of delayed onset GA-induced ALF requiring liver transplantation.
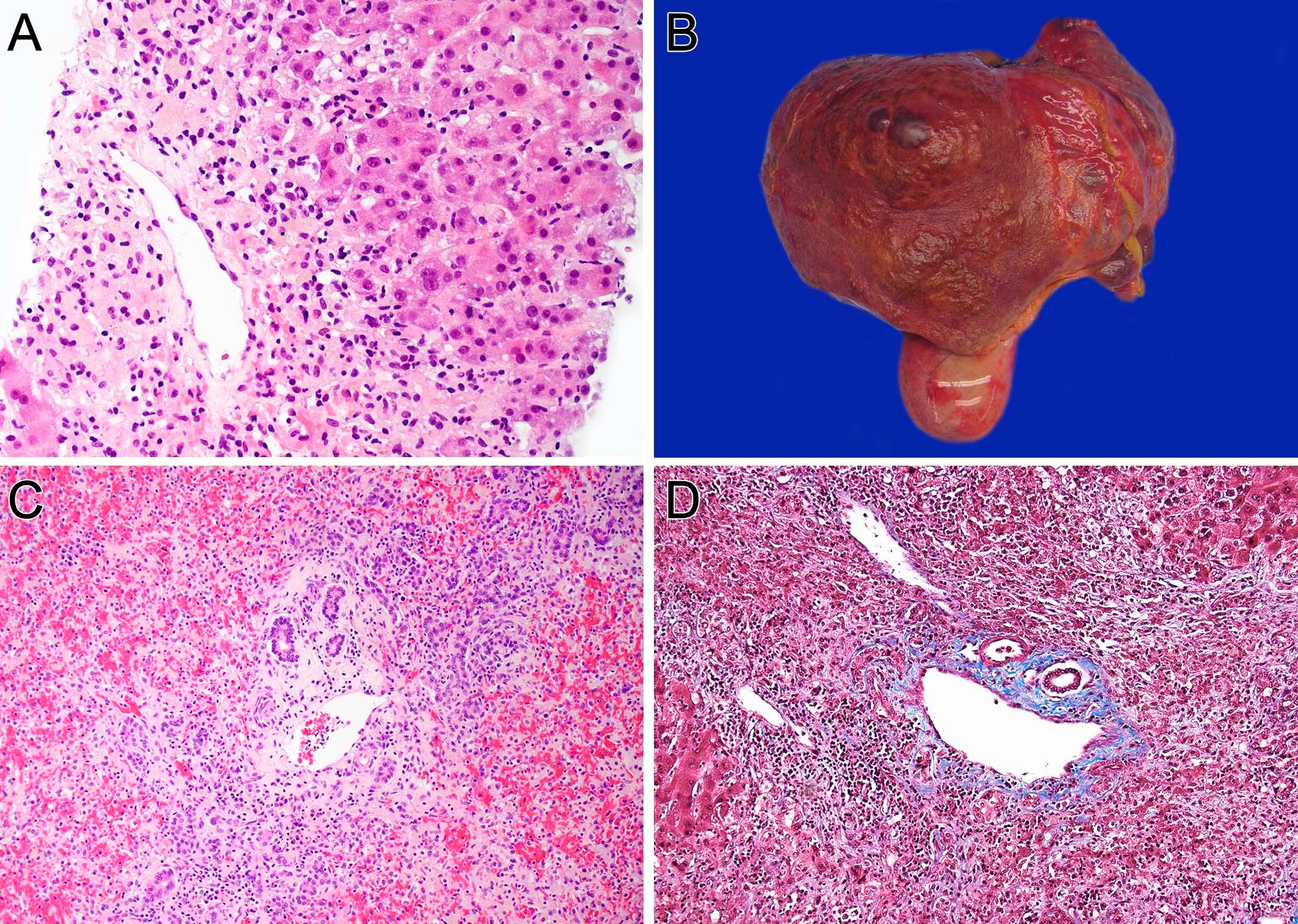
Case Description/Methods: A 59-year-old woman with well-controlled MS presented to the emergency department with three weeks of jaundice and dark-colored urine. Initial labs showed AST 2527 IU/L (81x ULN), ALT 2512 IU/L (81x ULN), total bilirubin 22.7 mg/dL (19x ULN), ALP 210 IU/L (2x ULN), INR 2.65. Further workup showed normal ceruloplasmin, alpha-1-antitrypsin, and IgG. Hepatitis, HSV, VZV, T-spot, HIV, ANA, anti-mitochondrial antibody, anti-LKM antibody were negative. Anti-smooth muscle antibody was 1:40. Her liver enzymes were normal one year prior to presentation, and she had no family history of autoimmune or liver disease. Computed tomography abdomen/pelvis showed patent vasculature and no evidence of cirrhosis. MRI/MRCP showed no biliary obstruction. Her only medication was GA, which she had been taking since 2008. Physical exam was notable for mild asterixis, and she was started on IV N-acetyl cysteine for 3 days. Liver biopsy demonstrated submassive necrosis (70%) consistent with drug induced liver injury (Fig1A). GA was thought to be the most likely etiology of her liver injury and was held. Liver transplant was ultimately deferred because her liver enzymes and her mental status improved. She was discharge on day 6.
Four days later she developed severe abdominal pain, lethargy and re-presented to the hospital. She was slow to respond, had scleral icterus and asterixis with AST 909 IU/L, ALT 839 IU/L, total bilirubin 27.2 mg/dL, INR 2.94. The patient underwent expedited liver transplant evaluation and was listed on the same day. She successfully underwent deceased donor liver transplant three days later. Liver explant showed mixed zone 1 and 3 liver necrosis (50%) with associated inflammation, mild lobular inflammation and cholestasis (Fig1B-D).
Discussion: Our case illustrates the first case of GA-induced ALF requiring liver transplantation. Previous reports showed that patients with GA drug-induced-liver injury recovered completely in 1-5 months after drug withdrawal and time of presentation ranges from 1 to 8 months, as opposed to 14 years in our case. Patients on GA should have long term regular liver monitoring.
Disclosures:
Diep Edwards, MD, Christine Lin, BS, Jessica Lin, MD, Kiyoko Oshima, MD, Elizabeth King, MD, Russell Wesson, MD, Peng-sheng Ting, MD, Shane Ottmann, MD, Ahmet Gurakar, MD. D0580 - Delayed Onset Drug-Induced Acute Liver Failure Caused by Glatiramer Acetate (GA) in Multiple Sclerosis Requiring Liver Transplantation, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Johns Hopkins School of Medicine, Baltimore, MD
Introduction: Glatiramer acetate (GA) has been used for multiple sclerosis (MS) since 1996. Regular liver function test monitoring is not required for the medication because there have not been any reported cases of liver toxicity.1Here, we report the first case of delayed onset GA-induced ALF requiring liver transplantation.
Case Description/Methods: A 59-year-old woman with well-controlled MS presented to the emergency department with three weeks of jaundice and dark-colored urine. Initial labs showed AST 2527 IU/L (81x ULN), ALT 2512 IU/L (81x ULN), total bilirubin 22.7 mg/dL (19x ULN), ALP 210 IU/L (2x ULN), INR 2.65. Further workup showed normal ceruloplasmin, alpha-1-antitrypsin, and IgG. Hepatitis, HSV, VZV, T-spot, HIV, ANA, anti-mitochondrial antibody, anti-LKM antibody were negative. Anti-smooth muscle antibody was 1:40. Her liver enzymes were normal one year prior to presentation, and she had no family history of autoimmune or liver disease. Computed tomography abdomen/pelvis showed patent vasculature and no evidence of cirrhosis. MRI/MRCP showed no biliary obstruction. Her only medication was GA, which she had been taking since 2008. Physical exam was notable for mild asterixis, and she was started on IV N-acetyl cysteine for 3 days. Liver biopsy demonstrated submassive necrosis (70%) consistent with drug induced liver injury (Fig1A). GA was thought to be the most likely etiology of her liver injury and was held. Liver transplant was ultimately deferred because her liver enzymes and her mental status improved. She was discharge on day 6.
Four days later she developed severe abdominal pain, lethargy and re-presented to the hospital. She was slow to respond, had scleral icterus and asterixis with AST 909 IU/L, ALT 839 IU/L, total bilirubin 27.2 mg/dL, INR 2.94. The patient underwent expedited liver transplant evaluation and was listed on the same day. She successfully underwent deceased donor liver transplant three days later. Liver explant showed mixed zone 1 and 3 liver necrosis (50%) with associated inflammation, mild lobular inflammation and cholestasis (Fig1B-D).
Discussion: Our case illustrates the first case of GA-induced ALF requiring liver transplantation. Previous reports showed that patients with GA drug-induced-liver injury recovered completely in 1-5 months after drug withdrawal and time of presentation ranges from 1 to 8 months, as opposed to 14 years in our case. Patients on GA should have long term regular liver monitoring.

Figure: Figure 1. Histological analysis of pre-transplant and explant liver. A. Hematoxylin and eosin (H&E) stain of the pre-transplant biopsy showed extensive necrosis including zone 3 (x100). B. Gross picture of explant (weight 500.3 gram). The surface showed wrinkles, which were characteristics of acute liver failure with massive hepatocyte necrosis. C. H&E stain of explant showed no viable hepatocytes in the worst area. (x200). D. Masson trichrome stain showed no fibrosis, which confirms the acute process (x200).
References
1. Biolato M, Bianco A, Lucchini M, Gasbarrini · Antonio, Mirabella M, Grieco · Antonio. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs. 123AD;35:861-880. doi:10.1007/s40263-021-00842-9
References
1. Biolato M, Bianco A, Lucchini M, Gasbarrini · Antonio, Mirabella M, Grieco · Antonio. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs. 123AD;35:861-880. doi:10.1007/s40263-021-00842-9
Disclosures:
Diep Edwards indicated no relevant financial relationships.
Christine Lin indicated no relevant financial relationships.
Jessica Lin indicated no relevant financial relationships.
Kiyoko Oshima indicated no relevant financial relationships.
Elizabeth King indicated no relevant financial relationships.
Russell Wesson indicated no relevant financial relationships.
Peng-sheng Ting indicated no relevant financial relationships.
Shane Ottmann indicated no relevant financial relationships.
Ahmet Gurakar indicated no relevant financial relationships.
Diep Edwards, MD, Christine Lin, BS, Jessica Lin, MD, Kiyoko Oshima, MD, Elizabeth King, MD, Russell Wesson, MD, Peng-sheng Ting, MD, Shane Ottmann, MD, Ahmet Gurakar, MD. D0580 - Delayed Onset Drug-Induced Acute Liver Failure Caused by Glatiramer Acetate (GA) in Multiple Sclerosis Requiring Liver Transplantation, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
