Back


Poster Session A - Sunday Afternoon
Category: Pediatrics
A0601 - Real World Ustekinumab Use in Pediatric Patients With Crohn’s Disease
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio
- TR
Timothy E. Ritter, MD
GI Alliance
Southlake, TX
Presenting Author(s)
Timothy E. Ritter, MD1, Harry E. Sarles, MD, MACG2, Annette E. Whitney, MD3, Dawn N. Kim-Romo, PharmD, PhD4, Lucinda J. Van Anglen, PharmD4
1GI Alliance, Southlake, TX; 2Digestive Health Associates of Texas/GI Alliance, Rockwall, TX; 3Digestive Health Associates of Texas, Plano, TX; 4Healix Infusion Therapy, LLC, Sugar Land, TX
Introduction: Ustekinumab (UST), a humanized IgG1κ monoclonal antibody antagonist of interleukin-12 and interleukin-23, is currently approved for patients (pts) 18+ years with moderate-to-severe Crohn’s disease (CD). Promising clinical results have been reported in the literature with UST in pediatric CD pts. The purpose of this study was to evaluate the real-world experience of UST in pediatric CD pts treated at large gastrointestinal private practices.
Methods: This was a retrospective observational analysis of CD pts aged ≤18 years who initiated UST therapy. Pts were followed for a minimum of 1 year, and additional analyses were conducted for those with 104 weeks of follow-up data. Demographics, disease characteristics, previous therapy, UST utilization, and adverse events data were collected from electronic medical records. The short pediatric Crohn’s disease activity index (sPCDAI) was used to assess disease activity at UST initiation, 6 weeks, 24 weeks, 52 weeks, and 104 weeks. Clinical remission was based on sPDCAI scores < 15. Corticosteroid (CS) use was evaluated at each time point.
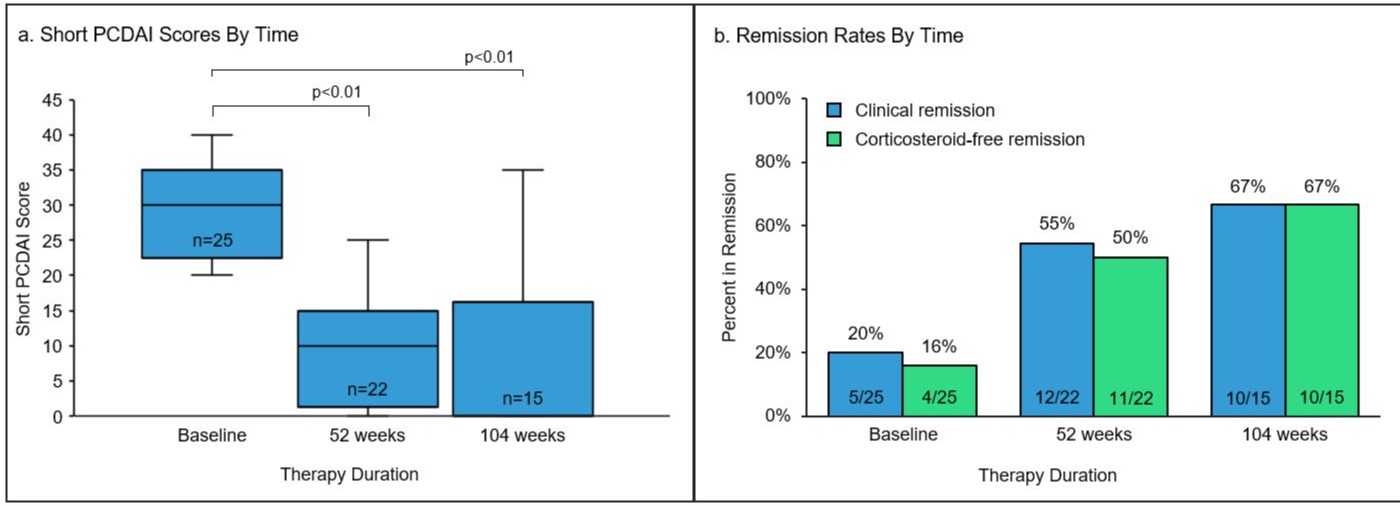
Results: There were 25 eligible pts (48% female) who initiated UST therapy during the study period. The mean (SD) age and disease duration was 16.5 (2.3) years and 3.3 (2.8) years, respectively. The median [IQR] weight was 62.1 [45-69] kg, and the majority (52%) used a UST intravenous induction dose of 390mg. All pts subsequently received UST 90mg subcutaneously every 4-8 weeks. Most (88%) were biologic-experienced having failed one (n=8), two (=13), or three (n=1) biologic therapies previously. Three pts were biologic-naïve. Of the 25 pts, 22 (88%) had follow-up data available at 52 weeks and 15 pts (60%) at 104 weeks. Figure 1 displays the significant reductions in sPCDAI scores compared to baseline. At 52 weeks, 12 out of 22 pts (55%) had reached clinical remission, and 11 of 22 pts (50%) were in CS-free remission. By week 104, the 10 out of 15 pts (67%) who reached clinical remission were also CS-free. Within the first 52 weeks, 3 pts discontinued UST due to tolerability (n=1 at induction) or the lack or loss of response (n=2).
Discussion: Pediatric pts experienced significant improvement in disease activity scores at both 52 and 104 weeks. Our long-term UST data shows good rates of clinical and CS-free remission in pediatric CD pts. Further studies in this population are warranted.
Disclosures:
Timothy E. Ritter, MD1, Harry E. Sarles, MD, MACG2, Annette E. Whitney, MD3, Dawn N. Kim-Romo, PharmD, PhD4, Lucinda J. Van Anglen, PharmD4. A0601 - Real World Ustekinumab Use in Pediatric Patients With Crohn’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1GI Alliance, Southlake, TX; 2Digestive Health Associates of Texas/GI Alliance, Rockwall, TX; 3Digestive Health Associates of Texas, Plano, TX; 4Healix Infusion Therapy, LLC, Sugar Land, TX
Introduction: Ustekinumab (UST), a humanized IgG1κ monoclonal antibody antagonist of interleukin-12 and interleukin-23, is currently approved for patients (pts) 18+ years with moderate-to-severe Crohn’s disease (CD). Promising clinical results have been reported in the literature with UST in pediatric CD pts. The purpose of this study was to evaluate the real-world experience of UST in pediatric CD pts treated at large gastrointestinal private practices.
Methods: This was a retrospective observational analysis of CD pts aged ≤18 years who initiated UST therapy. Pts were followed for a minimum of 1 year, and additional analyses were conducted for those with 104 weeks of follow-up data. Demographics, disease characteristics, previous therapy, UST utilization, and adverse events data were collected from electronic medical records. The short pediatric Crohn’s disease activity index (sPCDAI) was used to assess disease activity at UST initiation, 6 weeks, 24 weeks, 52 weeks, and 104 weeks. Clinical remission was based on sPDCAI scores < 15. Corticosteroid (CS) use was evaluated at each time point.
Results: There were 25 eligible pts (48% female) who initiated UST therapy during the study period. The mean (SD) age and disease duration was 16.5 (2.3) years and 3.3 (2.8) years, respectively. The median [IQR] weight was 62.1 [45-69] kg, and the majority (52%) used a UST intravenous induction dose of 390mg. All pts subsequently received UST 90mg subcutaneously every 4-8 weeks. Most (88%) were biologic-experienced having failed one (n=8), two (=13), or three (n=1) biologic therapies previously. Three pts were biologic-naïve. Of the 25 pts, 22 (88%) had follow-up data available at 52 weeks and 15 pts (60%) at 104 weeks. Figure 1 displays the significant reductions in sPCDAI scores compared to baseline. At 52 weeks, 12 out of 22 pts (55%) had reached clinical remission, and 11 of 22 pts (50%) were in CS-free remission. By week 104, the 10 out of 15 pts (67%) who reached clinical remission were also CS-free. Within the first 52 weeks, 3 pts discontinued UST due to tolerability (n=1 at induction) or the lack or loss of response (n=2).
Discussion: Pediatric pts experienced significant improvement in disease activity scores at both 52 and 104 weeks. Our long-term UST data shows good rates of clinical and CS-free remission in pediatric CD pts. Further studies in this population are warranted.

Figure: Figure 1. Disease Activity Scores and Remission Rates in Pediatric Crohn's Disease Patients on Ustekinumab
Disclosures:
Timothy Ritter: AbbVie – Advisor or Review Panel Member. Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Eli Lilly – Advisor or Review Panel Member. Ferring Pharmaceuticals – Advisor or Review Panel Member. Genetech – Advisor or Review Panel Member. Gilead Sciences – Advisor or Review Panel Member. Gossamer Bio – Advisor or Review Panel Member. Intercept Pharmaceuticals – Advisor or Review Panel Member. Janssen Pharmaceuticals – Advisor or Review Panel Member, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Prometheus Biosciences – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau.
Harry Sarles indicated no relevant financial relationships.
Annette Whitney indicated no relevant financial relationships.
Dawn Kim-Romo indicated no relevant financial relationships.
Lucinda Van Anglen: Ferring Pharmaceuticals – Advisor or Review Panel Member.
Timothy E. Ritter, MD1, Harry E. Sarles, MD, MACG2, Annette E. Whitney, MD3, Dawn N. Kim-Romo, PharmD, PhD4, Lucinda J. Van Anglen, PharmD4. A0601 - Real World Ustekinumab Use in Pediatric Patients With Crohn’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
