Back

Poster Session B - Monday Morning
Category: Liver
B0602 - Newfound Glow: The First Reported Case of Drug-Induced Liver Injury Due to Tofacitinib
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
.jpg)
Carlo S. Basilio, MD, MPH
Tulane University School of Medicine
New Orleans, LA
Presenting Author(s)
Carlo S. Basilio, MD, MPH, Samantha Wu, MD, John Bloch, MD, MPH, Hanadi Osman, MD, Tong Wu, MD, PhD, Martin Moehlen, MD, MPH
Tulane University School of Medicine, New Orleans, LA
Introduction: Tofacitinib, a Janus kinase (JAK) inhibitor, is a medication used to treat autoinflammatory conditions. It has previously been associated with mild hepatocellular liver enzyme elevations, but here we describe the first reported case of a clinically apparent drug-induced liver injury (DILI) related to tofacitinib.
Case Description/Methods: A 29-year-old man with ulcerative colitis developed jaundice, malaise, anorexia, abdominal pain, diarrhea, and pruritus six months after starting tofacitinib. His only other medication was lisinopril. He consumes less than three alcoholic drinks weekly. Liver chemistries revealed aspartate aminotransferase (AST) 635 IU/L, alanine aminotransferase (ALT) 614 IU/L, alkaline phosphatase (ALP) 150 IU/L, total bilirubin (T-Bili) 3.2 mg/dL, and direct bilirubin (D-Bili) 1.7 mg/dL. Coagulation studies were normal. Abdominal ultrasound showed a diffusely hypoechoic liver and a diffusely thickened gallbladder wall but no biliary pathology. He stopped taking tofacitinib, and his liver chemistries peaked at AST 826 IU/L, ALT 1427 IU/L, ALP 182 IU/L, and T-Bili 6.5 mg/dL. Anti-smooth muscle antibody was slightly positive at 41. Additional workup included negative antinuclear antibody and negative serologies for cytomegalovirus, Epstein-Barr virus, and hepatitis A, B, and C. His symptoms improved, and he resumed tofacitinib two months after symptom onset.
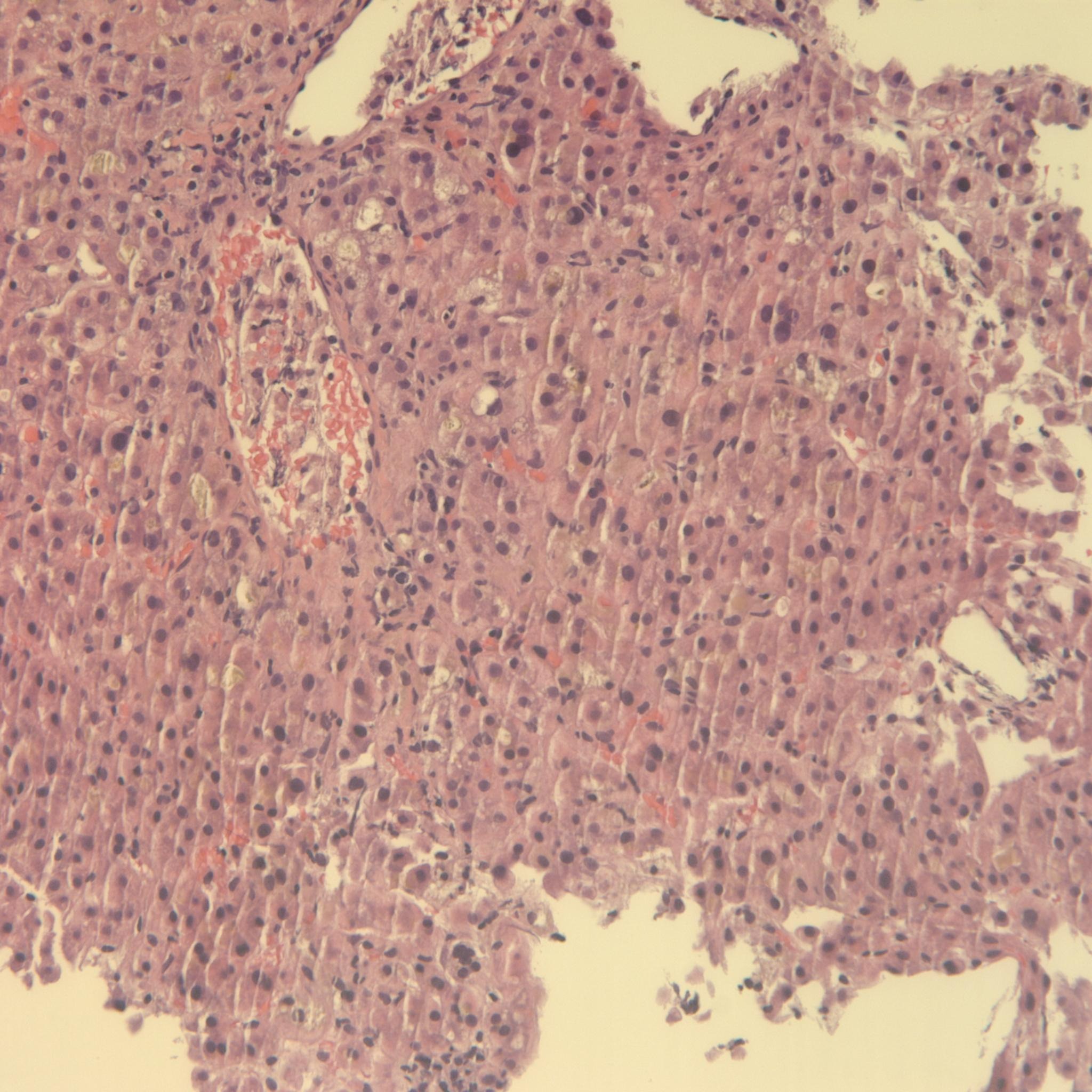
Four months later, the patient’s symptoms recurred and labs redemonstrated acute liver injury with a new peak of T-Bili 9.1 mg/dL. Liver biopsy revealed acute hepatitis with diffuse severe hepatocanalicular cholestasis, inflammation, and necrosis, consistent with DILI. He stopped taking tofacitinib, and after one month his symptoms resolved and liver chemistries normalized. He was transitioned to vedolizumab and has remained asymptomatic.
Discussion: This patient developed a clinically apparent liver injury with jaundice six months after initiating therapy with tofacitinib for ulcerative colitis. Other causes, such as viral hepatitis, alcohol-related liver disease, and autoimmune hepatitis, were ruled out. Biopsy results were consistent with DILI, and he was on no other culprit medications. He was rechallenged with tofacitinib and redeveloped liver injury, strongly supporting the diagnosis of tofacitinib-induced DILI. The mechanism of liver injury is not fully understood but might be attributed to drug metabolism via CYP3A4. More research should be conducted to better elucidate the mechanism of liver injury by tofacitinib.
Disclosures:
Carlo S. Basilio, MD, MPH, Samantha Wu, MD, John Bloch, MD, MPH, Hanadi Osman, MD, Tong Wu, MD, PhD, Martin Moehlen, MD, MPH. B0602 - Newfound Glow: The First Reported Case of Drug-Induced Liver Injury Due to Tofacitinib, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Tulane University School of Medicine, New Orleans, LA
Introduction: Tofacitinib, a Janus kinase (JAK) inhibitor, is a medication used to treat autoinflammatory conditions. It has previously been associated with mild hepatocellular liver enzyme elevations, but here we describe the first reported case of a clinically apparent drug-induced liver injury (DILI) related to tofacitinib.
Case Description/Methods: A 29-year-old man with ulcerative colitis developed jaundice, malaise, anorexia, abdominal pain, diarrhea, and pruritus six months after starting tofacitinib. His only other medication was lisinopril. He consumes less than three alcoholic drinks weekly. Liver chemistries revealed aspartate aminotransferase (AST) 635 IU/L, alanine aminotransferase (ALT) 614 IU/L, alkaline phosphatase (ALP) 150 IU/L, total bilirubin (T-Bili) 3.2 mg/dL, and direct bilirubin (D-Bili) 1.7 mg/dL. Coagulation studies were normal. Abdominal ultrasound showed a diffusely hypoechoic liver and a diffusely thickened gallbladder wall but no biliary pathology. He stopped taking tofacitinib, and his liver chemistries peaked at AST 826 IU/L, ALT 1427 IU/L, ALP 182 IU/L, and T-Bili 6.5 mg/dL. Anti-smooth muscle antibody was slightly positive at 41. Additional workup included negative antinuclear antibody and negative serologies for cytomegalovirus, Epstein-Barr virus, and hepatitis A, B, and C. His symptoms improved, and he resumed tofacitinib two months after symptom onset.
Four months later, the patient’s symptoms recurred and labs redemonstrated acute liver injury with a new peak of T-Bili 9.1 mg/dL. Liver biopsy revealed acute hepatitis with diffuse severe hepatocanalicular cholestasis, inflammation, and necrosis, consistent with DILI. He stopped taking tofacitinib, and after one month his symptoms resolved and liver chemistries normalized. He was transitioned to vedolizumab and has remained asymptomatic.
Discussion: This patient developed a clinically apparent liver injury with jaundice six months after initiating therapy with tofacitinib for ulcerative colitis. Other causes, such as viral hepatitis, alcohol-related liver disease, and autoimmune hepatitis, were ruled out. Biopsy results were consistent with DILI, and he was on no other culprit medications. He was rechallenged with tofacitinib and redeveloped liver injury, strongly supporting the diagnosis of tofacitinib-induced DILI. The mechanism of liver injury is not fully understood but might be attributed to drug metabolism via CYP3A4. More research should be conducted to better elucidate the mechanism of liver injury by tofacitinib.

Figure: Liver biopsy with acute hepatitis, severe hepatocanalicular cholestasis, inflammation, and necrosis, as well as a multinucleated hepatocyte, consistent with drug-induced liver injury in the setting of tofacitinib use.
Disclosures:
Carlo Basilio indicated no relevant financial relationships.
Samantha Wu indicated no relevant financial relationships.
John Bloch indicated no relevant financial relationships.
Hanadi Osman indicated no relevant financial relationships.
Tong Wu indicated no relevant financial relationships.
Martin Moehlen indicated no relevant financial relationships.
Carlo S. Basilio, MD, MPH, Samantha Wu, MD, John Bloch, MD, MPH, Hanadi Osman, MD, Tong Wu, MD, PhD, Martin Moehlen, MD, MPH. B0602 - Newfound Glow: The First Reported Case of Drug-Induced Liver Injury Due to Tofacitinib, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
