Back

Poster Session E - Tuesday Afternoon
Category: Functional Bowel Disease
E0264 - Potential of Tenapanor as a Treatment for Chronic Idiopathic Constipation (CIC): A Post Hoc Analysis From the T3MPO-1 and T3MPO-2 Phase 3 Studies for Irritable Bowel Syndrome With Constipation (IBS-C) in Adults
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Lucinda A. A. Harris, MS, MD
Mayo Clinic, Alix School of Medicine
Scottsdale, Arizona
Presenting Author(s)
Lucinda A. Harris, MS, MD1, David P. Rosenbaum, PhD2
1Mayo Clinic, Alix School of Medicine, Scottsdale, AZ; 2Ardelyx, Inc., Waltham, MA
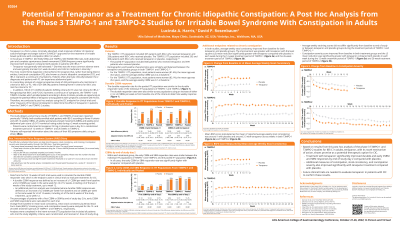
Introduction: Tenapanor is a first-in-class, minimally absorbed, small-molecule inhibitor of the intestinal sodium/hydrogen exchanger isoform 3 (NHE3), approved for the treatment of IBS-C in adults. Most recently ROME IV diagnostic criteria recognize that rather than being distinct entities functional constipation (FC) and IBS-C represent a continuum of symptoms. Wong, et al (AJG 2010) demonstrated that 89.5 % of patients meeting ROME III criteria for IBS-C also met the criteria for FC. Recognizing this overlap we performed a post hoc analysis, evaluating the efficacy of tenapanor for FC by utilizing the primary endpoint for chronic idiopathic constipation (CIC) in patients with IBS-C from the T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) phase 3 studies.
Am J Gastroenterol 2010; 105:2228–2234 Wong RK et al.
Methods: Adult patients with IBS-C (ROME III) with < 3 weekly complete spontaneous bowel movements (CSBM), ≤5 weekly spontaneous bowel movements (SBM) and weekly worst abdominal pain score ≥3 (0-10 numerical rating scale) during a 2-week screening period were randomized to tenapanor 50 mg or placebo twice a day. Data from the first 12 weeks of both trials was used to calculate the durable responder rate, which is the regulatory endpoint for CIC. A durable responder is a patient with a weekly increase of ≥1 CSBM from baseline and ≥3 CSBM per week for 9 of 12 weeks and 3 of the last 4 weeks of study.
Results: In the T3MPO-1 ITT population (tenapanor, n=307; placebo, n=299; mean age 45 years; 81% female), the responder rate vs placebo in durable responder rate was 11.28% (adjusted relative risk 3.35; p < 0.001). In the T3MPO-2 ITT population (tenapanor, n=293; placebo, n=300; mean age 45 years; 82% female), the responder rate vs placebo in durable responder rate was 15.49% (adjusted relative risk 3.75; p < 0.001).
Tenapanor was generally well tolerated. Diarrhea was the most common adverse event; severe diarrhea was reported in 2.5% of tenapanor-treated patients.
Discussion: Based on results from this post hoc analysis of the T3MPO-1 and T3MPO-2 Phase 3 studies for IBS-C in adults, tenapanor, with its novel mechanism of action, shows promise as a potential therapeutic option for CIC. Further clinical trials are needed to evaluate tenapanor for the CIC population to confirm these results.
Disclosures:
Lucinda A. Harris, MS, MD1, David P. Rosenbaum, PhD2. E0264 - Potential of Tenapanor as a Treatment for Chronic Idiopathic Constipation (CIC): A Post Hoc Analysis From the T3MPO-1 and T3MPO-2 Phase 3 Studies for Irritable Bowel Syndrome With Constipation (IBS-C) in Adults, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mayo Clinic, Alix School of Medicine, Scottsdale, AZ; 2Ardelyx, Inc., Waltham, MA
Introduction: Tenapanor is a first-in-class, minimally absorbed, small-molecule inhibitor of the intestinal sodium/hydrogen exchanger isoform 3 (NHE3), approved for the treatment of IBS-C in adults. Most recently ROME IV diagnostic criteria recognize that rather than being distinct entities functional constipation (FC) and IBS-C represent a continuum of symptoms. Wong, et al (AJG 2010) demonstrated that 89.5 % of patients meeting ROME III criteria for IBS-C also met the criteria for FC. Recognizing this overlap we performed a post hoc analysis, evaluating the efficacy of tenapanor for FC by utilizing the primary endpoint for chronic idiopathic constipation (CIC) in patients with IBS-C from the T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) phase 3 studies.
Am J Gastroenterol 2010; 105:2228–2234 Wong RK et al.
Methods: Adult patients with IBS-C (ROME III) with < 3 weekly complete spontaneous bowel movements (CSBM), ≤5 weekly spontaneous bowel movements (SBM) and weekly worst abdominal pain score ≥3 (0-10 numerical rating scale) during a 2-week screening period were randomized to tenapanor 50 mg or placebo twice a day. Data from the first 12 weeks of both trials was used to calculate the durable responder rate, which is the regulatory endpoint for CIC. A durable responder is a patient with a weekly increase of ≥1 CSBM from baseline and ≥3 CSBM per week for 9 of 12 weeks and 3 of the last 4 weeks of study.
Results: In the T3MPO-1 ITT population (tenapanor, n=307; placebo, n=299; mean age 45 years; 81% female), the responder rate vs placebo in durable responder rate was 11.28% (adjusted relative risk 3.35; p < 0.001). In the T3MPO-2 ITT population (tenapanor, n=293; placebo, n=300; mean age 45 years; 82% female), the responder rate vs placebo in durable responder rate was 15.49% (adjusted relative risk 3.75; p < 0.001).
Tenapanor was generally well tolerated. Diarrhea was the most common adverse event; severe diarrhea was reported in 2.5% of tenapanor-treated patients.
Discussion: Based on results from this post hoc analysis of the T3MPO-1 and T3MPO-2 Phase 3 studies for IBS-C in adults, tenapanor, with its novel mechanism of action, shows promise as a potential therapeutic option for CIC. Further clinical trials are needed to evaluate tenapanor for the CIC population to confirm these results.
Disclosures:
Lucinda Harris: AbbVie – Advisory Committee/Board Member. Allakos – Grant/Research Support. Alnylam – Advisory Committee/Board Member. Ardelyx – Advisory Committee/Board Member. Gemelli Biotech – Advisory Committee/Board Member. Ironwood – Consultant. Salix – Advisory Committee/Board Member. takeda – Grant/Research Support.
David Rosenbaum: Ardelyx, Inc. – Employee.
Lucinda A. Harris, MS, MD1, David P. Rosenbaum, PhD2. E0264 - Potential of Tenapanor as a Treatment for Chronic Idiopathic Constipation (CIC): A Post Hoc Analysis From the T3MPO-1 and T3MPO-2 Phase 3 Studies for Irritable Bowel Syndrome With Constipation (IBS-C) in Adults, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
