Back
Poster Session E - Tuesday Afternoon
E0563 - Liver Injury Caused by a Weight Loss Supplement Containing Green Tea Extract and Garcinia Cambogia
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Ankit Mishra, MS
Georgetown University School of Medicine
Washington, DC
Presenting Author(s)
Stephanie Woo, MD, Ankit Mishra, MS, James H. Lewis, MD, FACG
Georgetown University School of Medicine, Washington, DC
Introduction: Herbal-induced liver injury (HILI) is a growing topic worldwide, and herbal and dietary supplements (HDS) are the second leading class of compounds after antimicrobials causing liver injury in the United States. It is thought that many cases go unrecognized, as patients fail to mention their use of HDS. This is a case of mild liver injury in a young woman after taking a short course of a weight loss supplement containing a garcinia cambogia (GC) and green tea extract (GTE).
Case Description/Methods: A 45-year-old female with a history of obesity presented to clinic with elevated liver enzymes identified on routine labs from two weeks prior. Medication reconciliation revealed that one month prior, she started a 15-day course of Revert 10.0 by Natureal for weight loss. She stopped taking the supplement on her own as she felt it was not helpful. Her only prescription medication was her oral contraceptive pill. She was born in the United States and denied any prior history of liver disease.
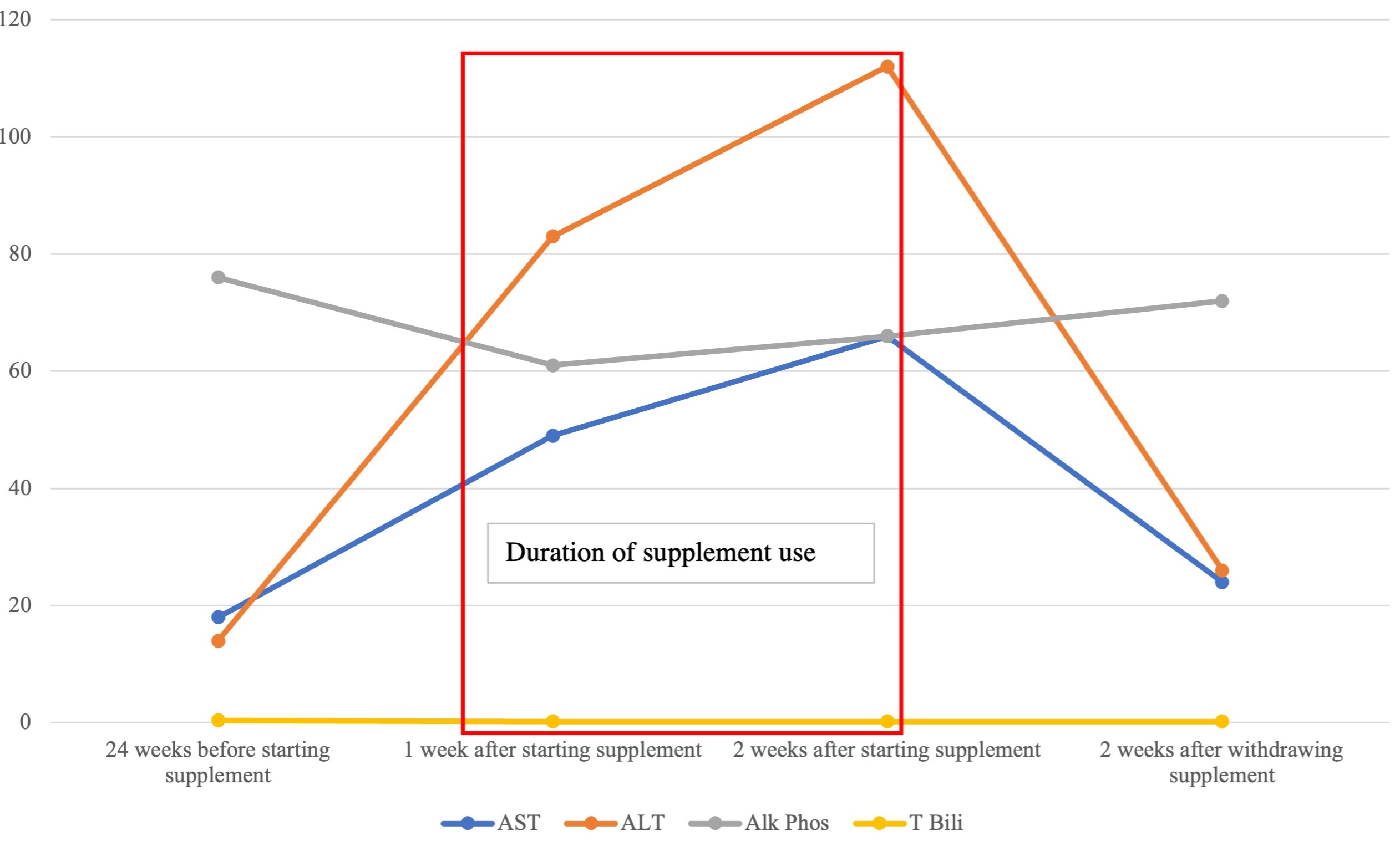
She was asymptomatic with no nausea, abdominal pain, or jaundice. Her physical exam was unremarkable. Baseline laboratory values from six months prior showed AST 18 U/L (normal < 40), ALT 14 U/L (normal < 32), ALP 76 U/L (normal 39-117), TBili 0.4 mg/dL (normal < 1.2). Her liver tests after one week of using Revert 10.0 revealed elevations of AST to 49 and ALT to 83, with normal ALP 61, and TBili 0.2. LFTs after two weeks showed further rises in AST to 66, and ALT to 112, with normal ALP 66, and TBili 0.2. Her viral hepatitis panel was non-reactive. An abdominal ultrasound and CT scan of the abdomen/pelvis showed no abnormalities with normal echogenicity and liver morphology. Two weeks after withdrawing the supplement, her LFTs had returned to normal, AST 24, ALT 26, ALP 72, and TBili 0.2.
Discussion: This is the first report of liver injury from Revert 10.0. We encourage clinicians to consider HILI in patients with even mild laboratory abnormalities, and to conduct thorough history-taking. While GC and GTE are both known hepatotoxins, there are no cases reported of the two agents working in conjunction to cause HILI. Furthermore, the Roussel Uclaf Causality Assessment Method, the gold standard for HILI diagnosis does not consider the potential for synergy between multiple known hepatotoxic agents. Our case highlights that further research should explore how synergy of multiple supplements can affect presentation of HILI.
Disclosures:
Stephanie Woo, MD, Ankit Mishra, MS, James H. Lewis, MD, FACG. E0563 - Liver Injury Caused by a Weight Loss Supplement Containing Green Tea Extract and Garcinia Cambogia, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Georgetown University School of Medicine, Washington, DC
Introduction: Herbal-induced liver injury (HILI) is a growing topic worldwide, and herbal and dietary supplements (HDS) are the second leading class of compounds after antimicrobials causing liver injury in the United States. It is thought that many cases go unrecognized, as patients fail to mention their use of HDS. This is a case of mild liver injury in a young woman after taking a short course of a weight loss supplement containing a garcinia cambogia (GC) and green tea extract (GTE).
Case Description/Methods: A 45-year-old female with a history of obesity presented to clinic with elevated liver enzymes identified on routine labs from two weeks prior. Medication reconciliation revealed that one month prior, she started a 15-day course of Revert 10.0 by Natureal for weight loss. She stopped taking the supplement on her own as she felt it was not helpful. Her only prescription medication was her oral contraceptive pill. She was born in the United States and denied any prior history of liver disease.
She was asymptomatic with no nausea, abdominal pain, or jaundice. Her physical exam was unremarkable. Baseline laboratory values from six months prior showed AST 18 U/L (normal < 40), ALT 14 U/L (normal < 32), ALP 76 U/L (normal 39-117), TBili 0.4 mg/dL (normal < 1.2). Her liver tests after one week of using Revert 10.0 revealed elevations of AST to 49 and ALT to 83, with normal ALP 61, and TBili 0.2. LFTs after two weeks showed further rises in AST to 66, and ALT to 112, with normal ALP 66, and TBili 0.2. Her viral hepatitis panel was non-reactive. An abdominal ultrasound and CT scan of the abdomen/pelvis showed no abnormalities with normal echogenicity and liver morphology. Two weeks after withdrawing the supplement, her LFTs had returned to normal, AST 24, ALT 26, ALP 72, and TBili 0.2.
Discussion: This is the first report of liver injury from Revert 10.0. We encourage clinicians to consider HILI in patients with even mild laboratory abnormalities, and to conduct thorough history-taking. While GC and GTE are both known hepatotoxins, there are no cases reported of the two agents working in conjunction to cause HILI. Furthermore, the Roussel Uclaf Causality Assessment Method, the gold standard for HILI diagnosis does not consider the potential for synergy between multiple known hepatotoxic agents. Our case highlights that further research should explore how synergy of multiple supplements can affect presentation of HILI.

Figure: Liver Enzyme Trend Over Time
Disclosures:
Stephanie Woo indicated no relevant financial relationships.
Ankit Mishra indicated no relevant financial relationships.
James Lewis indicated no relevant financial relationships.
Stephanie Woo, MD, Ankit Mishra, MS, James H. Lewis, MD, FACG. E0563 - Liver Injury Caused by a Weight Loss Supplement Containing Green Tea Extract and Garcinia Cambogia, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
