Back
Poster Session C - Monday Afternoon
C0571 - A Rare Case of Pembrolizumab-Induced Hepatitis
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Hunza Chaudhry, MD
UCSF-Fresno
Fresno, CA
Presenting Author(s)
Hunza Chaudhry, MD1, Joanne Lin, DO2, Aalam Sohal, MD2, Alakh Gulati, MD2, Marina Roytman, MD2
1UCSF-Fresno, Fresno, CA; 2UCSF Fresno, Fresno, CA
Introduction: The recent use of immune checkpoint inhibitors (ICI) has contributed to major breakthroughs in cancer therapy. Pembrolizumab is a monoclonal antibody used for many solid tumors. We report a case of a patient with gastric adenocarcinoma who developed drug-induced liver injury (DILI) from pembrolizumab.
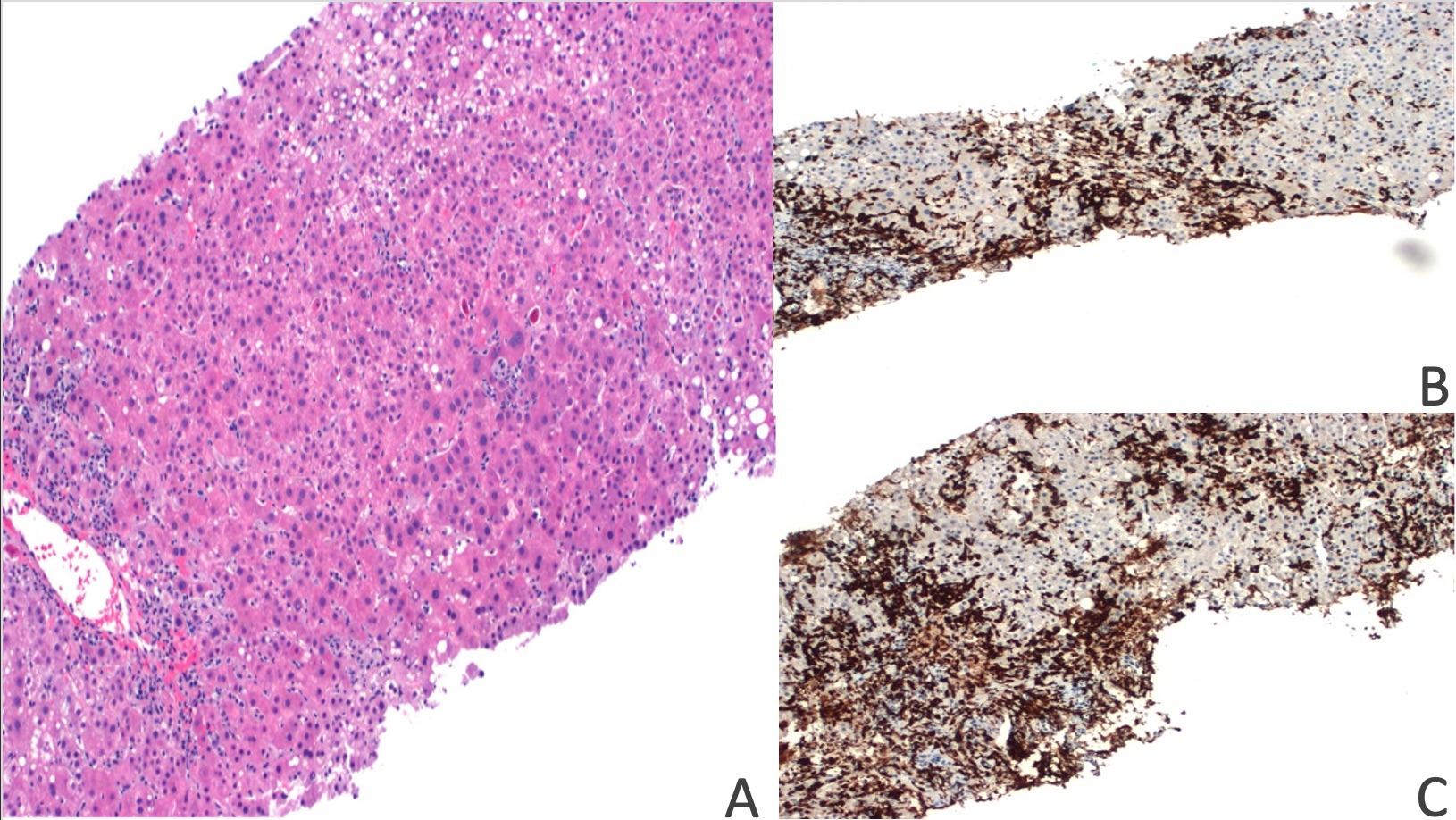
Case Description/Methods: A 64-year-old woman with a history of latent tuberculosis on isoniazid and gastric adenocarcinoma undergoing pembrolizumab immunotherapy presented for elevated liver enzymes noticed during an outpatient visit. The patient was asymptomatic, but laboratory values revealed AST (aspartate transaminase) 227 U/L, ALT (alanine transaminase) 494 U/L, alkaline phosphatase 217 U/L, and total bilirubin 1.4 mg/dL. Complete blood count (CBC) was unremarkable. She had received seven doses of pembrolizumab, with the last dose being four weeks before admission. Computed tomography (CT) of the abdomen and magnetic resonance cholangiopancreatography (MRCP) were unremarkable. Considering a RUCAM (Roussel Uclaf Causality Assessment Method) score of 5 and a possibility for DILI, liver biopsy was pursued. Biopsy results revealed small clusters of plasma cells (CD3+/CD8+) and numerous apoptotic hepatocytes with evidence of confluent centrizonal necrosis (Figure 1). Given the immunohistochemical staining pattern, a diagnosis of immune checkpoint inhibitor hepatitis was considered more likely. Pembrolizumab was discontinued, methylprednisolone and ursodiol were initiated. Patient’s liver enzymes improved with discontinuation of pembrolizumab and transition of isoniazid to another anti-tubercular agent. Ultimately, pembrolizumab was resumed outpatient with close monitoring.
Discussion: Pembrolizumab is a highly selective, humanized monoclonal antibody that inhibits lymphocytes' PD-1 receptors, allowing an immune response against cancer cells. The risk of liver injury is higher when combined with other hepatotoxic drugs, as demonstrated in our patient. Treatment consists of suspected medication cessation and immunosuppressants. Following cessation of ICI’s, liver enzymes usually normalize within weeks, as seen in our patient. Although not required for diagnosis, a liver biopsy can help rule out other etiologies. As ICIs become more widespread in the fight against malignancies, recognition of their adverse effects can lead to early diagnosis, intervention, and initiation of life-saving treatment.
Disclosures:
Hunza Chaudhry, MD1, Joanne Lin, DO2, Aalam Sohal, MD2, Alakh Gulati, MD2, Marina Roytman, MD2. C0571 - A Rare Case of Pembrolizumab-Induced Hepatitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1UCSF-Fresno, Fresno, CA; 2UCSF Fresno, Fresno, CA
Introduction: The recent use of immune checkpoint inhibitors (ICI) has contributed to major breakthroughs in cancer therapy. Pembrolizumab is a monoclonal antibody used for many solid tumors. We report a case of a patient with gastric adenocarcinoma who developed drug-induced liver injury (DILI) from pembrolizumab.
Case Description/Methods: A 64-year-old woman with a history of latent tuberculosis on isoniazid and gastric adenocarcinoma undergoing pembrolizumab immunotherapy presented for elevated liver enzymes noticed during an outpatient visit. The patient was asymptomatic, but laboratory values revealed AST (aspartate transaminase) 227 U/L, ALT (alanine transaminase) 494 U/L, alkaline phosphatase 217 U/L, and total bilirubin 1.4 mg/dL. Complete blood count (CBC) was unremarkable. She had received seven doses of pembrolizumab, with the last dose being four weeks before admission. Computed tomography (CT) of the abdomen and magnetic resonance cholangiopancreatography (MRCP) were unremarkable. Considering a RUCAM (Roussel Uclaf Causality Assessment Method) score of 5 and a possibility for DILI, liver biopsy was pursued. Biopsy results revealed small clusters of plasma cells (CD3+/CD8+) and numerous apoptotic hepatocytes with evidence of confluent centrizonal necrosis (Figure 1). Given the immunohistochemical staining pattern, a diagnosis of immune checkpoint inhibitor hepatitis was considered more likely. Pembrolizumab was discontinued, methylprednisolone and ursodiol were initiated. Patient’s liver enzymes improved with discontinuation of pembrolizumab and transition of isoniazid to another anti-tubercular agent. Ultimately, pembrolizumab was resumed outpatient with close monitoring.
Discussion: Pembrolizumab is a highly selective, humanized monoclonal antibody that inhibits lymphocytes' PD-1 receptors, allowing an immune response against cancer cells. The risk of liver injury is higher when combined with other hepatotoxic drugs, as demonstrated in our patient. Treatment consists of suspected medication cessation and immunosuppressants. Following cessation of ICI’s, liver enzymes usually normalize within weeks, as seen in our patient. Although not required for diagnosis, a liver biopsy can help rule out other etiologies. As ICIs become more widespread in the fight against malignancies, recognition of their adverse effects can lead to early diagnosis, intervention, and initiation of life-saving treatment.

Figure: Figure 1: A. H&E stain showing apoptotic hepatocytes and centrizonal vein with perivenular inflammation and hepatocellular dropout. B&C. Immunohistochemical staining positive for CD8 and CD3 lymphocytes.
Disclosures:
Hunza Chaudhry indicated no relevant financial relationships.
Joanne Lin indicated no relevant financial relationships.
Aalam Sohal indicated no relevant financial relationships.
Alakh Gulati indicated no relevant financial relationships.
Marina Roytman indicated no relevant financial relationships.
Hunza Chaudhry, MD1, Joanne Lin, DO2, Aalam Sohal, MD2, Alakh Gulati, MD2, Marina Roytman, MD2. C0571 - A Rare Case of Pembrolizumab-Induced Hepatitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

