Back
Poster Session C - Monday Afternoon
C0391 - Effectiveness and Safety of Weight Loss Medical Therapy in Ulcerative Colitis
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Jonathan Pham, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Jonathan Pham, MD, Amanda Johnson, MD
Mayo Clinic, Rochester, MN
Introduction: The prevalence of obesity in ulcerative colitis (UC) parallels that of the general population and may negatively impact UC outcomes. Options for obesity management in UC have not been well studied. Given the sparse data regarding their use, we sought to review the effectiveness and safety of weight loss medications in the UC population.
Methods: UC patients with prior use of FDA-approved weight loss medication (liraglutide, semaglutide, orlistat, phentermine-topiramate, phentermine, bupropion-naltrexone) were identified via the electronic medical record at Mayo Clinic between January 2001 and January 2022. Retrospective chart review was performed to obtain demographic data, UC and obesity-related history, as well as weight loss, safety, and UC outcomes. UC flare was defined as change or escalation in UC therapy, corticosteroid use, hospitalization, or surgery. Descriptive statistics were utilized for all outcomes.
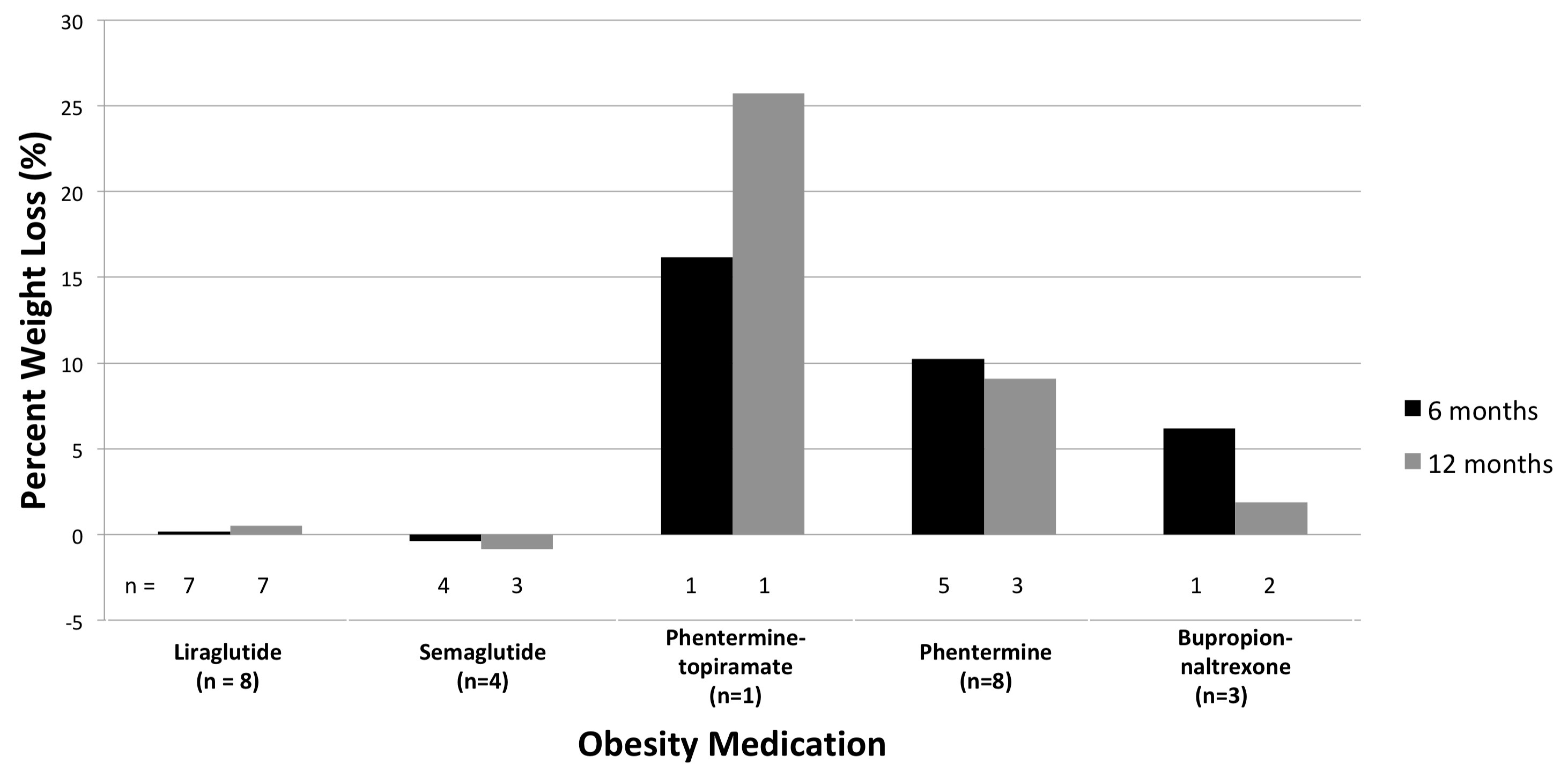
Results: Twenty-four patients were identified across five FDA-approved weight loss therapies. No patients were identified using orlistat. At baseline, twenty patients (83%) were in clinical remission and seven (29%) were treated with a biologic therapy. The liraglutide, phentermine-topiramate, phentermine, and bupropion-naltrexone groups demonstrated weight loss when evaluating the mean percent weight loss in those with follow-up at 12 months (0.5%, 25.7%, 9.1%, 1.9% respectively) (Figure 1). Six liraglutide patients (75%) received diabetes dosing. Those receiving adequate obesity dosing achieved 3.2% mean weight loss. Five patients (21%) reported side effects – one of which resulted in therapy discontinuation (Table 1). One serious adverse event, a cerebrovascular accident, occurred in the phentermine group. A UC flare occurred in two patients receiving semaglutide, both requiring corticosteroids, and one resulting in a change of maintenance therapy. No patients required hospitalization or surgery.
Discussion: While the liraglutide (particularly those receiving obesity-approved doses), phentermine-topiramate, phentermine, and bupropion-naltrexone groups all lost weight, only those using phentermine-topiramate and phentermine achieved >5% weight loss at 12 months. Reported side effects appeared similar to those of the general population. Although further studies are required to evaluate the efficacy of obesity therapy in UC, it may be a viable adjunct to lifestyle modification, especially in patients who do not qualify for bariatric surgery.
Disclosures:
Jonathan Pham, MD, Amanda Johnson, MD. C0391 - Effectiveness and Safety of Weight Loss Medical Therapy in Ulcerative Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Mayo Clinic, Rochester, MN
Introduction: The prevalence of obesity in ulcerative colitis (UC) parallels that of the general population and may negatively impact UC outcomes. Options for obesity management in UC have not been well studied. Given the sparse data regarding their use, we sought to review the effectiveness and safety of weight loss medications in the UC population.
Methods: UC patients with prior use of FDA-approved weight loss medication (liraglutide, semaglutide, orlistat, phentermine-topiramate, phentermine, bupropion-naltrexone) were identified via the electronic medical record at Mayo Clinic between January 2001 and January 2022. Retrospective chart review was performed to obtain demographic data, UC and obesity-related history, as well as weight loss, safety, and UC outcomes. UC flare was defined as change or escalation in UC therapy, corticosteroid use, hospitalization, or surgery. Descriptive statistics were utilized for all outcomes.
Results: Twenty-four patients were identified across five FDA-approved weight loss therapies. No patients were identified using orlistat. At baseline, twenty patients (83%) were in clinical remission and seven (29%) were treated with a biologic therapy. The liraglutide, phentermine-topiramate, phentermine, and bupropion-naltrexone groups demonstrated weight loss when evaluating the mean percent weight loss in those with follow-up at 12 months (0.5%, 25.7%, 9.1%, 1.9% respectively) (Figure 1). Six liraglutide patients (75%) received diabetes dosing. Those receiving adequate obesity dosing achieved 3.2% mean weight loss. Five patients (21%) reported side effects – one of which resulted in therapy discontinuation (Table 1). One serious adverse event, a cerebrovascular accident, occurred in the phentermine group. A UC flare occurred in two patients receiving semaglutide, both requiring corticosteroids, and one resulting in a change of maintenance therapy. No patients required hospitalization or surgery.
Discussion: While the liraglutide (particularly those receiving obesity-approved doses), phentermine-topiramate, phentermine, and bupropion-naltrexone groups all lost weight, only those using phentermine-topiramate and phentermine achieved >5% weight loss at 12 months. Reported side effects appeared similar to those of the general population. Although further studies are required to evaluate the efficacy of obesity therapy in UC, it may be a viable adjunct to lifestyle modification, especially in patients who do not qualify for bariatric surgery.

Figure: Figure 1: Percent weight loss at 6 and 12 months in ulcerative colitis patients
| Liraglutide (n=8) | Semaglutide (n=4) | Phentermine-topiramate (n=1) | Phentermine (n=8) | Bupropion-naltrexone (n=3) | |
| Dose and duration of use | |||||
| Maximum dose, mg | 1.2 (2/8) 1.8 (4/8) 3.0 (2/8) | 0.5 SQ (2/4) 1.0 SQ (1/4) 14 oral (1/4) | 15-50 BID | 15 (1/8) 30 (1/8) 37.5 (6/8) | 16-180 (1/3) 25-150 (1/3) 32-360 (1/3) |
| Continued use at 12 months, n (%) | 7 (87.5) | 3 (75) | 1 (100) | 3 (37.5) | 2 (66.7) |
| Medication-Related Adverse Events | |||||
| Side effects, n | 1 | 1 | 0 | 3 | 0 |
| Description of side effects | Diarrhea | Diarrhea | - | Insomnia, constipation, headache | - |
| Drug discontinuation due to side effects, n | 1 | 0 | - | 0 | - |
| Serious adverse events (SAEs), n | 0 | 0 | 0 | 1 | 0 |
| Description of SAE | - | - | - | CVA | - |
| UC-Related Complications | |||||
| UC Flare1, n CS use Change in IBD therapy Hospitalization Surgery | 0 0 0 0 0 | 2 2 1 0 0 | 0 0 0 0 0 | 0 0 0 0 0 | 0 0 0 0 0 |
Table: Table 1: Safety of obesity pharmacotherapy in ulcerative colitis patients.
CVA: cerebrovascular accident; UC: ulcerative colitis; CS: corticosteroid
1: UC flare defined as increase/change in medication for worsening symptoms or objective evidence of inflammation on endoscopy/imaging, prescription of steroids for flare, hospitalization, or surgery
CVA: cerebrovascular accident; UC: ulcerative colitis; CS: corticosteroid
1: UC flare defined as increase/change in medication for worsening symptoms or objective evidence of inflammation on endoscopy/imaging, prescription of steroids for flare, hospitalization, or surgery
Disclosures:
Jonathan Pham indicated no relevant financial relationships.
Amanda Johnson indicated no relevant financial relationships.
Jonathan Pham, MD, Amanda Johnson, MD. C0391 - Effectiveness and Safety of Weight Loss Medical Therapy in Ulcerative Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

